Official Newsjournal of the Illinois Council of Health-System Pharmacists

November 2021
Volume 47 Issue 4
Columns
Professional Affairs - Call for Entries
Features
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
More
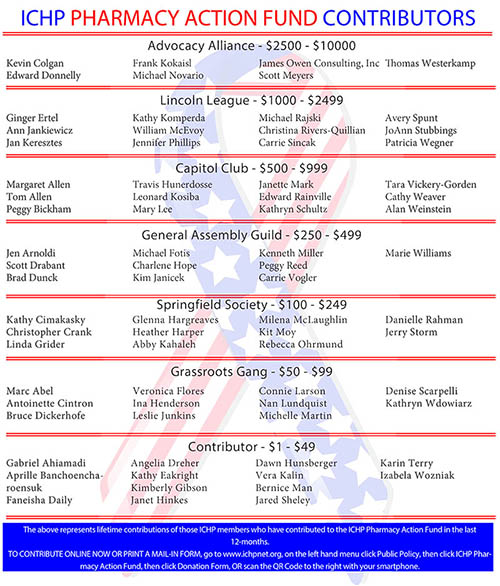
ICHP Pharmacy Action Fund (PAC)
Officers and Board of Directors
KeePosted Info

Illinois Council of Health-System Pharmacists
4055 North Perryville Road
Loves Park, IL 61111-8653
Phone: (815) 227-9292
Fax: (815) 227-9294
ichpnet.org
KeePosted
Official News journal of the Illinois Council of Health-System Pharmacists
EDITOR
Jennifer Phillips
ASSISTANT EDITOR
Milena McLaughlin
MANAGING EDITOR
Scott Meyers
ASSISTANT MANAGING EDITOR
Trish Wegner
DESIGN EDITOR
Melissa Dyrdahl
ICHP Staff
EXECUTIVE VICE PRESIDENT
Scott Meyers
VICE PRESIDENT - PROFESSIONAL SERVICES
Trish Wegner
DIRECTOR OF OPERATIONS
Maggie Allen
INFORMATION SPECIALIST
Heidi Sunday
CUSTOMER SERVICE AND
PHARMACY TECH TOPICS™ SPECIALIST
Jo Ann Haley
ACCOUNTANTS
Jan Mark
COMMUNICATIONS MANAGER
Melissa Dyrdahl
LEGISLATIVE CONSULTANT
Jim Owen
ICHP's Mission Statement
Advancing Excellence in Pharmacy
ICHP's Vision Statement
ICHP dedicates itself to achieving a vision of pharmacy practice where:
· Pharmacists are universally recognized as health care professionals and essential providers of health care services.
· Pharmacists use their medication expertise and leadership skills to optimize the medication use process and patient outcomes.
· Pharmacy technicians are trained and PTCB certified to manage the medication distribution process.
ICHP's Goal Statements
· Raising awareness of the critical role pharmacists fulfill in optimizing medication therapy and ensuring medication safety in team-based, patient-centered care.
· Providing high quality educational services through innovative continuing pharmacy education and training programs, and sharing evidence-based best practices.
· Developing and nurturing leaders through mentorship, skill development programs, and leadership opportunities.
· Working with national and state legislators and policymakers to create or revise legislation and regulation critical to pharmacy practice and quality patient care.
· Urging pharmacy technician employers to require successful completion of an accredited pharmacy technician training program and PTCB certification of all pharmacy technicians.
Approved by the ICHP Board of Directors May 30, 2018.
KeePosted Vision
As an integral publication of the Illinois Council of Health-System
Pharmacists, the KeePosted newsjournal will reflect its
mission and goals. In conjunction with those goals, KeePosted will
provide timely information that meets the changing professional and personal
needs of Illinois pharmacists and technicians, and maintain high publication
standards.
KeePosted is an official publication of, and is copyrighted by, the
Illinois Council of Health-System Pharmacists (ICHP). KeePosted is
published 10 times a year. ICHP members received KeePosted as
a member benefit. All articles published herein represent the opinions of the
authors and do not reflect the policy of the ICHP or the authors’ institutions
unless specified. Advertising inquiries can be directed to ICHP office at the
address listed above. Image disclaimer: The image used in the Pharmacy Tech
Topics™ advertisement is the property of © 2017 Thinkstock, a division of Getty
Images.
Copyright © 2018, Illinois Council of Health-System Pharmacists. All rights
reserved.
 Directly Speaking
Directly Speaking
There’s More to Life Than Pharmacy
by Scott A. Meyers, Executive Vice President
Pharmacy has been a big part of my life but it is not my life. I got into pharmacy to help people and anyone who says pharmacists don’t help people sit in the corner and think about it. But I have found that I can help people in many other ways than just helping them get better outcomes from their medications. And so can you!
Over the last year and a half, ICHP has tried to guide our members to support specific charitable organizations. This year we’re focused on the American Heart Association (AHA), a great organization with a strong history fighting one of the biggest killers each year - heart disease. We are encouraging members to walk with AHA in Rockford, Oak Brook, and Carterville. Check our website for more details. Members can also sponsor walkers or just donate directly to the AHA.
But there’s more ways to help and we certainly don’t want to dictate which charitable efforts you support. In fact, we encourage each and every one of you to seek out a volunteer opportunity that satisfies your own interest, cause, or concern. And if there is an opportunity to share your experience with others, let us know at the ICHP office and we’ll post the event on our Community Service page of the ICHP website! Did you know we have one? It can be found under the members’ tab on the home page.
So that means if you need sponsors for your marathon run, 5K walk, or local paint-a-thon, let us know in advance and we’ll see if we can’t drum up some support! If you have raw energy and no place to burn it, take a look at the Community Service page and maybe you’ll find a new passion.
For many years, my church held a volunteer event every June (including this year) called Sharefest, which started out painting, cleaning and renovating Rockford Public Schools. I volunteered for a few years, increasing my participation a little each year until I was asked to become a team leader. I learned a lot, met a ton of great people, and had great personal experiences that still generate warm memories. But I also learned to paint fairly well. Something pharmacists don’t learn in school but something we all eventually deal with personally or through someone we hire. The accomplishment of a nicely painted room can also provide a rewarding feeling especially if you never thought you would do it.
During my volunteer experiences I’ve done a lot of things that I never dreamed of doing and learned a lot along the way. But more importantly, I’ve gained great friends, helped others less fortunate, and generated fond memories. If you haven’t got a regular volunteer job that you enjoy regularly, this summer is a great time to look for one.
Volunteering relieves stress by taking your mind off your problems and focusing on the needs of others. Volunteering helps you meet new people, including some who may help you build a professional network. Volunteering gets you out of the house, away from your home computer or cell phone (at least you have to put it down when you’re working) and more often than not gets you some much needed exercise.
Sure, it can cost you a little in travel expenses and supplies (sometimes) but the ROI is amazing! And if you can’t physically participate, you can always donate. And if you are the recipient of sponsorship donations, don’t forget to report back to all those who donated with the results and the highlights of how the event went! It’s great to hear marathon times and exciting moments or see pictures of paint-splattered workers with big smiles and speckled faces.
Take volunteering seriously and you will be surprised what fun you can have! With just over two years until my retirement from ICHP, I’ve already begun thinking of new ways to add to my volunteer resume. I’m sure Sharefest will be a part of it along with the other church related positions I already have but my guess is that I will join my wife on Wednesdays at the local food pantry and maybe find my own gig with the Rockford Park District or a local homeless shelter. It doesn’t take much effort to identify our communities’ needs and only a little more effort to jump in and start meeting them. There is more to life than pharmacy!
 President's Message
President's Message
Start Asking Questions
by Travis Hunerdosse, PharmD, MBA, ICHP President
Have you considered questioning a skill that could be developed? Or how your own answers to questions impact conversation? If you are not asking questions, you may be missing an opportunity to make conversations more productive with your colleagues, boss, trainees, or preceptors. Asking questions, or questioning, fosters learning and idea exchange. Questioning provides energy for innovation, change, and process improvement. Asking the right questions in the right way can also expose breakdowns in systems that put an organization at risk. There are useful techniques that can be used in order to gain more from your questioning and how you answer others.
The act of conversation accomplishes two things; information exchange (learning) and impression management (liking). Asking questions achieves both. Questioning provides a channel for people to get to know one another and also demonstrates a genuine interest in the individual. Believe it or not, people liked to be asked questions. It makes them feel appreciated which in turn leaves a positive impression.
Consider this in context of a job interview. During a traditional interview, the candidate is the one answering most of the questions. They are also taking the opportunity to describe why they are the best candidate for the job, past experience, and qualifications. What leaves a lasting (and good) impression is when the candidate asks questions. Interviewees that ask questions demonstrate competence and an interest in the organization or the interviewer, develop a rapport, and learn key information that ensures that the position and the company are a good match.
In addition to beginning to ask questions, there are other factors that influence the quality of the conversation. These factors are type, tone, sequence, and framing. These can be taken into context when determining the desired outcome of the conversation. Active listening is also crucial. You are asking questions to gain information and learn. Also you want to be able to respond effectively to ensure you are achieving your goals in the conversation.
Consider the following techniques when approaching your next conversation:
- Follow up questions. Follow up questions demonstrate to the conversational partner that you are listening to their responses, care about what they have to contribute, and that you are interested in additional information. This type of question establishes a relationship where both parties feel mutually respected and heard. The best part of the follow up question is that it does not take much effort. They do not have to be prepared ahead of time or require complex thinking.
- Open ended questions. Use of open-ended questions keeps the conversation going and allow you to get the most information. Keeping questions open-ended sparks thinking and encourages people to open up to provide a wide array of responses. You may get an answer you were not expecting that unlocks a solution to a difficult problem. On the other hand, while your conversational partner does not want to get back into a corner by getting asked yes or no questions, sometimes you need to ask more direct questions to get the response you are looking for. Of course, it is important to keep these framed correctly so folks do not feel threatened.
- Sequence. The order in which you ask questions may vary depending on the type of conversation you are having. If your goal is to build relations with your conversational partner, start with less intrusive questions then escalate to more difficult ones to be most effective. However, if the encounter may be tense, it is best to start with the sensitive or tough questions first and then de-escalate. Used in tough situations, this tactic can make people more willing to open up.
- Tone. Your conversational partner will be more willing to open up when you ask questions in a casual manner versus a more formal approach. People are also more willing to answer questions when they are given “an out” or know they are able to change their answer based on additional questions. This holds true during brainstorming sessions. If participants are using a white board and are able to erase their answers, people are more willing to freely share ideas and less likely to hold back. Allowing information between individuals without inhibition leads to better learning and innovation.
Reference:
Wood Brooks, A., John, L.K. The Surprising Power of Questions. HBR. May-June, 2018. pg 60-67.
Columns
Board of Pharmacy Update
Highlights of the May Meeting
by Scott A. Meyers, Executive Vice President
NABP Annual Meeting – The National Association of Boards of Pharmacy conducted its Annual Meeting in Denver on May 5-8, 2018. Yash Patel served as the Illinois Delegate to the meeting with Al Carter serving as first alternate and Denise Scarpelli as second alternate. Hot topics of discussion and education included the opioid crisis, medical marijuana, technology including the use of social media by Boards of Pharmacy and USP compounding standards.
2018 District IV Meeting – Will be held in Grand Rapids, MI and hosted by Ferris State University College of Pharmacy and the Michigan Board of Pharmacy on November 7-9, 2018.
Department Update – Compounding rules comments are being reviewed for final publication and approval by JCAR. There is no specific date for approval at this time.
The license renewal process is nearly complete for pharmacists and pharmacies with over 90% of both received, however only approximately 69% of pharmacy technician renewals have been completed at the time of the meeting which appears to be low. There was no identified reason yet. Now would be a good time for all Pharmacists-In-Charge (PICs) to check to make sure their technicians have current licenses. Those without one should not be allowed to work, as the PIC will be disciplined also.
Legislative Update – The May Board Meeting update was scheduled to be presented by IPhA Executive Director, Garth Reynolds. However, he stayed in Springfield to testify on HB3479, so I provided this month’s update. The Spring Session of the General Assembly is well underway and there are a variety of bills impacting pharmacy. Several bills address the opioid crisis by amending the Controlled Substance Act. These and all the bills ICHP is monitoring are discussed in the Government Affairs Report in this issue of KeePosted.
Next Meeting – The next meeting of the Board is set for July 10, 2018 to begin at 10:30 am in the James R. Thompson Center at Randolph and LaSalle in downtown Chicago. These meetings are open to the public and pharmacists, pharmacy technicians, and pharmacy students are encouraged to attend.

Educational Affairs
New Oral Oncolytics of 2017: Key Points for a Counseling Pharmacist
by Janna Afanasjeva, PharmD, BCPSa; Chloe Majkowski, PharmD Candidate 2018a; Christopher Campbell, PharmD, BCPS, BCOPb; Institutional affiliations: aUniversity of Illinois Chicago; bNorthwestern Memorial Hospital
Abemaciclib (Verzenio)
Enasidenib (Idhifa)
Indication
Indication
Table 1. Key properties of newly approved oral oncology agents in 2017.6,11,16,20,23,26,29,31,33
|
Drug name (Brand) |
Dosage form, strength(s) |
Dosing |
Costa |
|
Abemaciclib (Verzenio™) |
Tablet; 50 mg, 100 mg, 150 mg, 200 mg |
200 mg twice daily as monotherapy; 150 mg twice daily in combination with fulvestrant 500 mg on Days 1, 15, 29, and then once monthly |
$3,284 (14 tablets)
|
|
Acalabrutinib (Calquence™) |
Capsule; 100 mg |
100 mg twice daily
|
$16,877 (60 capsules) |
|
Brigatinib (Alunbrig™) |
Tablet; 30 mg, 90 mg, 180 mg |
90 mg daily for 7 days, and then 180 mg daily if tolerated |
$1,995 (21 tablets) |
|
Enasidenib (Idhifa™) |
Tablet; 50 mg, 100 mg |
100 mg daily
|
$29,846 (30 tablets) |
|
Midostaurin (Rydapt™) |
Capsule; 25 mg
|
50 mg twice daily for AML; 100 mg twice daily for ASM, SM-AHN, or mast cell leukemia |
$4,497 (28 capsules) |
|
Niraparib (Zejula™) |
Capsule; 100 mg |
300 mg daily
|
$17,700 (90 capsules) |
|
Neratinib (Nerlynx™) |
Tablet; 40 mg
|
240 mg daily
|
$12,600 (180 tablets) |
|
Ribociclib (Kisqali™) |
Tablet; 200 mg
|
600 mg daily for 21 days, followed by 7 days off in combination with continuous letrozole |
$13,140 (63 tablets) |
- Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
- McCullough S, Newton R. Pharmacy's changing role as care transitions from infused to oral therapies. Am J Manag Care. 2017;23(12 Spec No.):sp468.
- Hematology/oncology (cancer) approvals & safety notifications. The Food and Drug Administration website. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Updated March 22, 2018. Accessed March 26, 2018.
- Bailey R, Newton R. PBMs: their role, the problems, and how practices can work with PBMs. Am J Manag Care. 2017;23(12 Spec No.):Sp497-sp498.
- The state of cancer care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract. 2017;13(4):e353-e394.
- Verzenio [package insert]. Indianapolis, IN: Eli Lilly and Company; 2018.
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 Inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218-5224.
- Sledge GW, Jr., Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646.
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Version 1.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Published March 20, 2018. Accessed March 26, 2018.
- Calquence [package insert]. Los Angeles, CA: Puma Biotechnology, Inc; 2017.
- Wang M, Rule S, Zinzani P, et al. Abstract 155: Efficacy and safety of acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma in the phase 2 ACE-LY-004 study. 2017 ASH Meeting. Available at: http://www.bloodjournal.org/content/130/Suppl_1/155. Accessed May 29, 2018.
- Imbruvica [package insert]. Sunnyvale, CA: Pharmacyclics LLC; 2017.
- NCCN Clinical Practice Guidelines in Oncology. B-Cell Lymphomas Version 2.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Published February 26, 2018. Accessed March 26, 2018.
- NCCN Clinical Practice Guidelines in Oncology. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Version 5.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Published March 26, 2018. Accessed April 11, 2018.
- Alunbrig [package insert]. Cambridge, MA: Ariad Pharmaceuticals, Inc; 2018.
- Camidge DR, Tiseo M, Ahn MJ, et al. Brigatinib in crizotinib-refractory ALK+ NSCLC: central assessment and updates from ALTA, a pivotal randomized phase 2 trial. J Thorac Oncol. 2016;12(1 suppl) [abstract P3.02a-013].
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer Version 3.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Published February 21, 2018. Accessed March 26, 2018.
- NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers Version 1.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Published March 20, 2018. Accessed April 11, 2018.
- Idhifa [package insert]. Summit, NJ: Celgene Corporation; 2017.
- Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
- NCCN Clinical Practice Guidelines in Oncology. Acute Myeloid Leukemia Version 1.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Published February 7, 2018. Accessed March 26, 2018.
- Rydapt [package insert]. East Hanover, NJ: Novartis; 2017.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464.
- Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541.
- Zejula [package insert]. Waltham, MA: Tesaro, Inc.; 2018.
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
- NCCN Clinical Practice Guidelines in Oncology. Ovarian Cancer Version 2.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Published March 9, 2018. Accessed March 26, 2018.
- Nerlynx [package insert]. Los Angeles, CA: Puma Biotechnology, Inc; 2017.
- Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367-377.
- Kisqali [package insert]. East Hanover, NJ: Novartis; 2017.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
- LexiComp [database]. Hudson, OH: Wolters Kluwer Health; 2018. https://online.lexi.com/lco/action/home. Accessed March 26, 2018
Leadership Profile
Meet Karin Terry, PharmD

Leadership position in ICHP
Director of Professional Affairs
Tell us about your position and practice site.
Medication Safety Pharmacist for OSF HealthCare. I am based in Peoria, but we have practice sites all over Central and Northern Illinois!
Tell us about a time you made a difference.
I will always remember this situation, because I was a new pharmacist and still in orientation at OSF-Saint Franice Medical Group. Xigris® had been approved the month before (I know, that dates me…), and our P&T had decided to add it to formulary just the day before. It was a Saturday in December, and I answered a call from an ICU attending who wanted Xigris® for a young septic patient. It was peak Christmas season, so I had a hard time finding someone who could help us order it. I ended up coordinating a drop shipment flight to get the drug there within 12 hours, doing “just in time” education with pharmacy and nursing staff, and keeping in touch with the attending all day. We got the patient the drug, and I got a personal “Thank You” note from the attending (whom I later learned was a very hard person to impress!). Now, I realize that Xigris® was later pulled from the market and may not have actually helped the patient…but the entire situation gave me so much confidence in my ability to handle a situation that seemed “above my paygrade”.
How did you know that pharmacy was for you?
I decided in 6th grade that I wanted to be a pharmacist. My brother had a “Careers” magazine that he brought home from high school. I looked up my favorite subjects, math and science, and one of the options was pharmacy. I boldly declared that I was going to be a pharmacist when I grew up. Stubbornly, I stuck to that declaration all through high school! Luckily for me, I ended up choosing a career that was a great fit for me. I love all the different places a pharmacy career can take you! Over the last almost 20 years, I have had some great experiences and met some amazing people.
Fill in the blank: ICHP is _____ because…
ICHP is vital, because it gives all of us an outlet to be able to see outside of ourselves and our current role. I have learned so much about what pharmacy can be by meeting pharmacists and techs from across the state in hospitals and clinics big and small. I also see ICHP’s role in advocacy as vital to our profession, and I love that it gives me easy access to get involved.
Tell us about when you first joined ICHP.
I have always been involved in pharmacy organizations since my time at Drake University. I knew that I wanted to be a member of ICHP once I started working at Saint Francis. Working at a facility with multiple pharmacists who were not just members but involved in committee and leadership positions definitely helped me stay engaged! It was easy to say that I was “too busy” with work and family to get involved in committees, but seeing my co-workers make it all happen and benefit from the relationships made me decide it was time to jump in!
Special thanks to so many people who have made me who I am today. My mom was a bedside nurse for 40 years. She always kept her passion for her care of her patients, and she showed me that you don’t have to have a title to earn respect. I had some amazing professors at Drake who made me look at pharmacy in a way I didn’t even know I could. There were a few specific preceptors during rotations and residency who greatly influenced how I saw myself as a pharmacist. I have had some very intelligent and motivated co-workers who keep me on my toes and push me to do more. And, of course, my family. I want my girls to know that I am working hard to help people.
What advice do you have for students?
Never exclude an area of pharmacy as a career option. I never would’ve thought I would be a Medication Safety Pharmacist. As a new grad, I would never have found my current role to be exciting enough. I wanted ICU or transplant or cardiology. But every little project I did that related to medication safety made me realize that I would really enjoy Medication Safety as a career. There are more areas in pharmacy than you realize, so keep your options open!
Any special interests or hobbies outside of work?
You will often find me in my “Mom uniform” at one of my daughters’ sporting events. Whether it is soccer, basketball, volleyball, or cross country, I love watching my girls do their thing!
For “me” time, I enjoy playing slow pitch softball with both my co-workers and my church league teams. I also enjoy running and training for the St Jude Run every August.
What is your favorite place to vacation?
We try to check out different places, but our favorite would have to be Park City, Utah. We were there for the bobsledding World Cup the year before the Salt Lake City Olympics, and we really enjoyed ourselves. Then we went back for the Winter Olympics the next year and we really fell in love with the place! They have great snow for snowboarding in the winter and great trails to hike, bike, and horseback in the summer.
Government Affairs Report
The List Gets A Little Shorter
by Jim Owen and Scott Meyers
As we enter the final month (May) of the spring session of the Illinois General Assembly, the list of active bills begins to shrink. Some of the losses are good but unfortunately, a few bills that have died would have been positive for pharmacy. One in particular, HB0274 would have allowed pharmacists with special training to prescribe hormonal contraceptives for their patients. Reluctantly, the sponsor will most assuredly bring this effort back next spring with the 101st General Assembly.
HB3479 is still breathing at the time of writing and would require managed care providers for Illinois’ Medicaid patients to reimburse pharmacies at the same rate as the fee-for-service Medicaid population. Currently community and outpatient pharmacies frequently receive less than the actual acquisition cost of the medication for payment and cannot refuse to fill those prescriptions or they will lose access to all Medicaid prescriptions. Losses have ranged from a few dollars to hundreds of dollars per prescription. If you’re wondering why we (ICHP) is concerned with this issue, it is two-fold. The first is that many of our members’ hospitals have outpatient pharmacies and are suffering and the second is to support the overall regulation and oversight of PBMs that currently have none in the State of Illinois.
While limitedly related to pharmacy in general, the budget battle wages on this year as it has in the past. It appears that Governor Rauner is not as steadfast on his reform platform with the hope of achieving a budget compromise before the end of May. It is unclear which solution will be acceptable, but it is fairly certain that Illinois’ long-term money woes will not be solved this session.
For a complete list of the bills that still have life at the time of this writing, they are provided below with a brief summary. We encourage you to visit the Illinois General Assembly website to get more information on any bill that draws your interest.
We also encourage you to find out who will be running for office this fall in your district. All State representative districts and 39 of the 59 State senate districts are up for election in the fall of 2018 so chances are you will have to make a decision or two this fall. Find out what the candidates’ stances are on health care and pharmacy so you are informed and ready to vote intelligently. In many districts, as is the case always in Illinois, you may not have a chance because your candidates are unopposed, but get to know them anyway. They will be representing you and the citizens of your district. Summer is a great time for that too with local fund raisers abounding. Bar-B-Ques, golf outings, corn boils and many other events will bring the candidates out to shake hands, kiss babies and raise re-election funds. Let us know if you attend a fund-raiser and what you learn from the candidate, it’s helpful intel.
Finally, if you’ve decided that it’s time to truly get into the advocacy game, this summer is a great time to make a contribution to the ICHP Pharmacy Action Fund, ICHP’s PAC, to support those legislators that listen to our concerns and see the real value of the pharmacist as a member of the health care team. Later this summer the PAC will be making contributions to help our friends in the General Assembly get re-elected so they can keep our voice heard and our message clear.
2018 Illinois General Assembly Bill Summary
|
Bill Number |
Sponsor |
Summary |
Location |
ICHP Position |
|
SB0336
Sam001
|
Harmon - Oak Park, D |
Amends SB336 by replacing everything after the enacting clause with the following: “Section 1. This Act may be referred to as the Alternatives to Opioids Act of 2018. Section 5. the Compassionate Use of Medical Cannabis Pilot Program Act is amended by changing sections 5, 10, 60, and 160 as follows…” Sam001 supporting cannabis as MAT for opioid use disorder |
Assigned to Executive Comm in the House
5/8/2018 |
|
|
SB1888 & HB3479 |
McCann- Jacksonville, R
Feigenholtz – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. In addition to other specified actions required under the Code, requires a managed care community network that contracts with the Department of Healthcare and Family Services to establish, maintain, and provide a fair and reasonable reimbursement rate to pharmacy providers for pharmaceutical services, prescription drugs and drug products, and pharmacy or pharmacist-provided services. Provides that the reimbursement methodology shall not be less than the current reimbursement rate utilized by the Department for prescription and pharmacy or pharmacist-provided services and shall not be below the actual acquisition cost of the pharmacy provider. Requires a managed care community network to ensure that the pharmacy formulary used by the managed care community network and its contract providers is no more restrictive than the Department's pharmaceutical program. Effective July 1, 2018. |
Assignments in Senate
5/5/2017
House Bill postponed in the Senate Human Services Comm.
5/2/2018 |
|
|
SB2226 |
Nybo - Lombard, R |
Amends the State Police Act. Provides that a physician, physician's assistant with prescriptive authority, or advanced practice registered nurse with prescriptive authority who provides a standing order or prescription for epinephrine auto-injectors in the name of the Department of State Police shall incur no civil or professional liability, except for willful and wanton conduct, as a result of any injury or death arising from the use of an epinephrine auto-injector. Amends the Illinois Police Training Act. Provides that a physician, physician's assistant with prescriptive authority, or advanced practice registered nurse with prescriptive authority who provides a standing order or prescription for epinephrine auto-injectors in the name of a local governmental agency shall incur no civil or professional liability, except for willful and wanton conduct, as a result of any injury or death arising from the use of an epinephrine auto-injector. Makes conforming changes to the Medical Practice Act of 1987 and the Public Health Standing Orders Act. Effective immediately. |
Judiciary – Civil Procedure Subccommittee in the House
5/9/2018
|
|
|
SB2524 SAM002
|
Rose - Champaign, R |
Sen. Amendment 1: Amends the Environmental Protection Act by creating the Pharmaceutical Disposal Task Force. The task force will coordinate a statewide public information campaign to highlight the benefits and opportunities of properly disposing of pharmaceutical products. Identifies the members of the task force and responsibilities.
Sen. Amendment 2: Added representatives of physician, coroner and pharmaceutical manufacturer to the task force. |
Passed in the Senate
2nd Reading. in the House
5/9/2018 |
Neutral |
|
SB2834 |
Syverson – Rockford, R |
Amends the Alcoholism and Other Drug Abuse and Dependency Act. Changes the short title of the Act to the Substance Use Disorder Act. Removes the terms "addict", "addiction", "alcoholic", "alcoholism", and "substance abuse" and their corresponding definitions. Requires the Department of Human Services to reduce the incidence of substance use disorders (rather than reduce the incidence and consequences of the abuse of alcohol and other drugs). Defines "substance use disorder". Requires the Department to design, coordinate, and fund prevention, early intervention, treatment, and other recovery support services for substance use disorders that are accessible and address the needs of at-risk individuals and their families. Requires the Department to develop a comprehensive plan on the provision of such services; assist other State agencies in developing and establishing substance use disorder services for the agencies' clients; adopt medical and clinical standards on how to determine a substance use disorder diagnosis; and other matters. Contains provisions concerning the licensing of substance use disorder treatment providers; licensure categories and services; the identification of individuals who need substance use disorder treatment using "SBIRT"; patients' rights; services for pregnant women, mothers, and criminal justice clients; and other matters. Repeals a provision of the Act establishing the Committee on Women's Alcohol and Substance Abuse Treatment. Repeals a provision of the Act setting forth the powers and duties of the Medical Advisory Committee. Makes conforming changes concerning the Substance Use Disorder Act to several Acts including the Department of Human Services Act, the Children and Family Services Act, and the Mental Health and Developmental Disabilities Administrative Act. Effective January 1, 2019. |
Rules Comm. in the House 4/27/2018 |
|
|
SB2889 |
Rose – Champaign, R |
Creates the Epinephrine Administration Act. Provides that a health care practitioner may prescribe epinephrine pre-filled syringes in the name of an authorized entity where allergens capable of causing anaphylaxis may be present. Provides that an authorized entity may acquire and stock a supply of undesignated epinephrine pre-filled syringes provided the undesignated epinephrine pre-filled syringes are stored in a specified location. Requires each employee, agent, or other individual of the authorized entity to complete a specified training program before using a pre-filled syringe to administer epinephrine. Provides that a trained employee, agent, or other individual of the authorized entity may either provide or administer an epinephrine pre-filled syringe to a person whom the employee, agent, or other individual believes in good faith is experiencing anaphylaxis. Provides that training under the Act shall be valid for 2 years. Requires the Department of Public Health to approve training programs, to list the approved programs on the Department's website, and to include links to training providers' websites on the Department's website. Contains provisions concerning costs, limitations, and rulemaking. Defines terms. Amends the School Code. In provisions concerning epinephrine administration, provides that epinephrine may be administered with a pre-filled syringe. Makes conforming changes. Senate Amendment 1: Changed “auto-injectors” to “injectors” Senate Amendment 2: Removes provision creating Epinephrine Administration Act. |
Human Services Comm. in the House
5/7/2018 |
|
|
SB2951 HB4950 |
Bush - Grayslake, D |
Creates the Early Mental Health and Addictions Treatment Act. Requires the Department of Healthcare and Family Services, and other specified agencies and entities, to develop a pilot program under which a qualifying adolescent or young adult may receive community-based mental health treatment from a youth-focused community support team for early treatment that is specifically tailored to the needs of youth and young adults in the early stages of a serious emotional disturbance or serious mental illness. Requires the Department to apply, no later than September 30, 2019, for any necessary federal waiver or State Plan amendment to implement the pilot program. Requires the Department to implement the pilot program no later than December 31, 2019 if federal approval is not necessary. Contains provisions concerning the creation of a community-based treatment model under the pilot program; the development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Requires the Department to develop an Assertive Engagement and Community-Based Clinical Treatment Pilot Program for individuals with opioid and other drug addictions. Contains provisions on in-office, in-home, and in-community services provided under the pilot program; application for a federal waiver or State Plan amendment to implement the pilot program; development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Effective immediately. |
Mental Health Comm. in the House
5/7/2018 |
|
|
SB2952 HB4907 |
Bush – Grayslake, D |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately |
Human Services Comm. in the House
05/08/2018 |
|
|
SB3015 |
Koehler - Peoria, D |
Amends the School Code. With regard to the self-administration and self-carry of asthma medication, provides that a school district, public school, charter school, or nonpublic school may authorize a school nurse or trained personnel to (i) provide undesignated asthma medication to a student for self-administration only or to any personnel authorized under a student's Individual Health Care Action Plan or asthma action plan, plan pursuant to Section 504 of the federal Rehabilitation Act of 1973, or individualized education program plan to administer to the student that meets the student's prescription on file, (ii) administer an undesignated asthma medication that meets the prescription on file to any student who has an Individual Health Care Action Plan or asthma action plan, plan pursuant to Section 504 of the federal Rehabilitation Act of 1973, or individualized education program plan that authorizes the use of asthma medication; and (iii) administer an undesignated asthma medication to any person that the school nurse or trained personnel believes in good faith is having respiratory distress; defines "undesignated asthma medication" and "respiratory distress". Changes the definition of "asthma medication" to mean quick-relief asthma medication that is approved by the United States Food and Drug Administration for the treatment of respiratory distress. Provides that a school nurse or trained personnel may administer undesignated asthma medication to any person whom the school nurse or trained personnel in good faith believes to be experiencing respiratory distress (i) while in school, (ii) while at a school-sponsored activity, (iii) while under the supervision of school personnel, or (iv) before or after normal school activities. Provides that a school district, public school, charter school, or nonpublic school may maintain a supply of an asthma medication in any secure location where a person is most at risk. Provides that a training curriculum to recognize and respond to respiratory distress may be conducted online or in person. Specifies training requirements. Makes other changes. Effective immediately. Senate Amendment 1: Added must notify the child’s health care provider AND school nurse within 24 hours after the administration of an undesignated asthma medication. |
Elementary & Secondary Education, School Curriculum & Policies Comm. in the House
5/7/2018 |
|
|
SB3116 |
Hunter – Chicago, D |
Amends the Nurse Practice Act. In provisions concerning written collaborative agreements, restores the ability of podiatric physicians to collaborate with advanced practice registered nurses. Makes other changes. Effective immediately. |
Health Care Licenses Comm. in the House
5/7/2018 |
|
|
SB3170 |
Stadelman - Rockford, D |
Amends the Pharmacy Practice Act and the Illinois Food, Drug and Cosmetic Act. Provides that a prescription for medication other than controlled substances shall be valid for up to 15 months from the date issued for the purpose of refills, unless the prescription states otherwise. |
Health Care Licenses Comm. in the House
5/2/2018 |
|
|
HB3479 SB1888 |
Feigenholtz – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. In addition to other specified actions required under the Code, requires a managed care community network that contracts with the Department of Healthcare and Family Services to establish, maintain, and provide a fair and reasonable reimbursement rate to pharmacy providers for pharmaceutical services, prescription drugs and drug products, and pharmacy or pharmacist-provided services. Provides that the reimbursement methodology shall not be less than the current reimbursement rate utilized by the Department for prescription and pharmacy or pharmacist-provided services and shall not be below the actual acquisition cost of the pharmacy provider. Requires a managed care community network to ensure that the pharmacy formulary used by the managed care community network and its contract providers is no more restrictive than the Department's pharmaceutical program. Effective July 1, 2018. |
Postponed in Human Services Comm. in the Senate
5/2/2018 |
Support |
|
HB4096 |
Harris – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. Provides that the Department of Healthcare and Family Services shall require each Medicaid Managed Care Organization to list as preferred on the Medicaid Managed Care Organization's preferred drug list every pharmaceutical that is listed as preferred on the Department's preferred drug list. Provides that the Department shall not prohibit, or adopt any rules or policies that prohibit, a Medicaid Managed Care Organization from: (i) covering additional pharmaceuticals that are not listed on the Department's preferred drug list; or (ii) removing from the Medicaid Managed Care Organization's preferred drug list any prior approval requirements applicable under the Department's preferred drug list. Provides that the Department shall not require a Medicaid Managed Care Organization to utilize a single, statewide preferred drug list and shall not prohibit a plan from negotiating drug pricing concessions or rebates on any drug with pharmaceutical companies, unless otherwise required by federal law. Provides that no later than July 1, 2018, the Department shall develop a standardized format for all Medicaid Managed Care Organization preferred drug lists in cooperation with Medicaid Managed Care Organizations and stakeholders, including, but not limited to, community-based organizations, providers, and individuals or entities with expertise in drug formulary development. Requires each Medicaid Managed Care Organization to post its preferred drug list on its website without restricting access to enrolled members and to update the preferred drug list posted on its website within 2 business days of making any changes to the preferred drug list, including, but not limited to, any and all changes to requirements for prior approval. Effective immediately. |
2nd Reading in the Senate
5/10/2018
|
|
|
HB4146 |
Fine – Glenview, D |
House Amendment 1 : n language providing that a health care plan is not prohibited from requiring a pharmacist to effect substitutions of prescription drugs, provides that the health care plan is not prohibited from requiring a pharmacist to effect substitutions consistent with provisions from the Pharmacy Practice Act that allow a pharmacist to substitute an interchangeable biologic for a prescribed biologic product and select a generic drug determined to be therapeutically equivalent by the United States Food and Drug Administration and in accordance with the Illinois Food, Drug and Cosmetic Act. |
Postponed Special Comm. on Oversight of Medicaid Managed Care in the Senate
5/2/2018
|
|
|
HB4331 |
Conner – Romeoville, D |
Amends the Counties Code. Provides that in every case in which an opioid overdose is determined to be a contributing factor in a death, the coroner shall report the death and the age, gender, race, and county of residence, if known, of the decedent to the Department of Public Health. Amends the University of Illinois Hospital Act and the Hospital Licensing Act. Requires every hospital to report the age, gender, race, and county of residence, if known, of each patient diagnosed as having an opioid overdose to the Department within 48 hours of the diagnosis. Amends the Department of Public Health Powers and Duties Law of the Civil Administrative Code of Illinois. Requires the Department to adopt rules to implement the reporting requirements. Requires the Department to annually report to the General Assembly the data collected. |
2nd Reading in the Senate
5/9/2018 |
|
|
HB4440 |
Gabel - Evanston, D |
Amends the Nursing Home Care Act. Provides that the Department of Public Health shall provide facilities with educational information on all vaccines recommended by the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, including, but not limited to, the risks associated with shingles and how to protect oneself against the varicella-zoster virus. Requires a facility to distribute the information to each resident who requests the information and each newly admitted resident. Allows the facility to distribute the information to residents electronically. Effective January 1, 2019. |
Placed on 3rd Reading in the Senate
5/2/2018 |
|
|
HB4643
|
Burke - Chicago, D |
Amends the Illinois Physical Therapy Act. Provides that the limitation on determining a differential diagnosis shall not in any manner limit a physical therapist from establishing a relevant diagnosis. In the definition of "documented current and relevant diagnosis" and in provisions concerning disciplinary actions, removes language requiring a diagnosis to be substantiated by a physician, dentist, advanced practice registered nurse, physician assistant, or podiatric physician. Effective immediately. |
2nd reading in the Senate
5/10/2018 |
|
|
HB4650
|
Zalewski - Riverside, D |
Amends the Illinois Controlled Substance Act. In a provision allowing pharmacists to authorize a designee to consult the Prescription Monitoring Program on their behalf, defines "pharmacist" to include, but be not limited to, a pharmacist associated with a health maintenance organization or a Medicaid managed care entity providing services under the Illinois Public Aid Code. Effective immediately. |
2nd reading in the Senate
5/10/2018
|
Oppose |
|
HB4707 House Amendment 001 |
Scherer - Decatur, D |
House Amendment 003: Creates the Prescription Drug Task Force Act and a Prescription drug task Force with 18 members, one member from ICHP that will study the extent of over prescribing of opioids to patients and to make recommendations for future legislation to address the issue. |
3rd reading in the Senate
5/10/2018 |
Neutral |
|
HB4795 |
Demmer – Rochelle, R |
Amends the Alcoholism and Other Drug Abuse and Dependency Act. Changes the short title of the Act to the Substance Use Disorder Act. Removes the terms "addict", "addiction", "alcoholic", "alcoholism", and "substance abuse" and their corresponding definitions. Requires the Department of Human Services to reduce the incidence of substance use disorders (rather than reduce the incidence and consequences of the abuse of alcohol and other drugs). Defines "substance use disorder". Requires the Department to design, coordinate, and fund prevention, early intervention, treatment, and other recovery support services for substance use disorders that are accessible and address the needs of at-risk individuals and their families. Requires the Department to develop a comprehensive plan on the provision of such services; assist other State agencies in developing and establishing substance use disorder services for the agencies' clients; adopt medical and clinical standards on how to determine a substance use disorder diagnosis; and other matters. Contains provisions concerning the licensing of substance use disorder treatment providers; licensure categories and services; the identification of individuals who need substance use disorder treatment using "SBIRT"; patients' rights; services for pregnant women, mothers, and criminal justice clients; and other matters. Repeals a provision of the Act establishing the Committee on Women's Alcohol and Substance Abuse Treatment. Repeals a provision of the Act setting forth the powers and duties of the Medical Advisory Committee. Makes conforming changes concerning the Substance Use Disorder Act to several Acts including the Department of Human Services Act, the Children and Family Services Act, and the Mental Health and Developmental Disabilities Administrative Act. Effective January 1, 2019. |
Placed on 3rd Reading in the Senate
5/8/2018 |
|
|
HB4900 |
Guzzardi - Chicago, D |
Creates the Illinois Generic Drug Pricing Fairness Act. Provides that a manufacturer or wholesale drug distributor shall not engage in price gouging in the sale of an essential off-patent or generic drug. Provides that the Director of Healthcare and Family Services or Director of Central Management Services may notify the Attorney General of any increase in the price of any essential off-patent or generic drug under the Medical Assistance Program under the Illinois Public Aid Code or a State health plan, respectively, that amounts to price gouging. Provides that whenever the Attorney General has reason to believe that a manufacturer or wholesale drug distributor of an essential off-patent or generic drug has violated the Act, the Attorney General shall send a notice to the manufacturer or wholesale drug distributor requesting a specified statement. Provides that within 45 days after receipt of the request, the manufacturer or wholesale drug distributor shall submit the statement to the Attorney General. Provides that to accomplish the objectives and carry out the duties prescribed in the Act, the Attorney General may issue subpoenas or examine under oath any person to determine whether a manufacturer or wholesale drug distributor has violated the Act. Provides that upon petition of the Attorney General, a circuit court may issue specified orders against violations of the Act. Contains provisions concerning the disclosure of financial information provided by a manufacturer or wholesale drug distributor to the Attorney General. Effective January 1, 2019. |
Passed in the House, in Assignments in the Senate
4/23/2018 |
|
|
HB4907 SB2952 |
McAuliffe - Chicago, R |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. House Amendment 1 now requires the designee in the pharmacy must be a licensed individual. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately |
Postponed in Public Health Comm. in the Senate
5/9/2018
|
|
|
HB5070 |
Bellock – Westmont, R |
Amends the Telehealth Act. Includes clinicians licensed to provide medical services under Illinois law in the definition of "health care professional". Amendment 001: Added pharmacists and other licensed practitioners under the definition of “health care professional”. |
Placed on 2nd Reading in the Senate
05/03/2018 |
|
|
HB5442 |
Durkin - Burr Ridge, R |
Amends the Open Meetings Act. Provides that, for the purposes of the Act, "public body" does not include a Metropolitan Enforcement Group (MEG) Policy Board or drug task force composed or created by any combination of local law enforcement agencies. Amends the Criminal Code of 2012. Provides that a person commits drug-induced homicide when he or she violates delivery of a controlled substance or methamphetamine or a similar law of another jurisdiction, by unlawfully delivering a controlled substance to another, and the injection, inhalation, absorption, or ingestion of any amount of that controlled substance is a contributing cause of the person's death. Amends the Illinois Controlled Substances Act. Provides that controlled substances which are lawfully administered in hospitals or institutions licensed under the Hospital Licensing Act shall be reported under (rather than, exempt from) specified reporting provisions under the Act, and the prescription for the controlled substances ordered and the quantity actually administered (rather than, the reporting requirement only applies for more than a 72-hour supply of a discharge medication to be consumed outside of the hospital or institution). Provides that the information required to be transmitted under the prescription monitoring program must be transmitted not later than the end of the business day on which a controlled substance is dispensed, or at such other time as may be required by the Department of Human Services by administrative rule (rather than, at the end of the next business day on which the controlled substance is dispensed). |
Tabled 04/12/2018
|
Neutral |
Senate Deadlines
New Practitioners Network
Ask For Advice
by Mary Lacy, PharmD, PGY-2 Health-System Pharmacy Administration Resident, University of Illinois at Chicago
Another misconception among new practitioners is that only experienced pharmacists can serve as mentors. All pharmacists have leadership responsibilities, whether they hold official leadership positions or not. As Sara White discusses in her “Leadership: Successful alchemy” article, there are two types of leaders – “big L” and “little L” leaders.1 “Big L” leaders are those in formal leadership positions, such as pharmacy directors, managers, and supervisors. Yet every pharmacist is a “little L”, guiding pharmacy technicians, pharmacy students, and even fellow pharmacists.
ICHPeople
Our members have a lot to celebrate!
Congratulations to our members from Midwestern University Chicago
College of Pharmacy on their promotions and achievements!
Promotion to Professor: Jen D’Souza (with Tenure)
Promotion to Associate Professor: Milena McLaughlin (with Tenure)
Promotion to Adjunct Associate Professor: Amy Lullo
2017-2018 Golden Apple Award: Carrie Sincak
2017-2018 Preceptor of the Year Award: Milena McLaughlin
2017-2018 New Preceptor Excellence Award: Andrew Merker
2017-2018 Preceptor of the Year Award for Adjunct Faculty: William Budris
Professional Affairs - Call for Entries

Past winners include:
2017
Sandra M. Salverson, Pharm.D., BCPS and Jerry Storm, RPh
Implementation of Integrated Telepharmacy Services Achieve a Health-System Standard of Pharmacy Care
2016
Maya Beganovic, PharmD and Sarah M. Wieczorkiewicz, PharmD, BCPS
"MALDI-TOF alone versus MALDI-TOF combined with real-time antimicrobial stewardship interventions on time to optimal therapy in patients with positive blood cultures"
2015
Kuntal Patel, Pharm.D., Pavel Prusakov, and Heather Vaule
"Osteopenia of Prematurity (aka Better Bones for Babies)"
2014
Arti Phatak, Pharm.D.; Brooke Ward, Pharm.D., BCPS; Rachael Prusi, Pharm.D.; Elizabeth Vetter, Pharm.D.; Michael Postelnick, BS Pharm, BCPS (AQ Infectious Diseases); and Noelle Chapman, Pharm.D., BCPS
"Impact of Pharmacist Involvement in the Transitional Care of High-Risk Patients through Medication Reconciliation, Medication Education, and Post-Discharge Callbacks"
View 2008 - 2013 Winners at ichpnet.org
Online entry form: http://www.ichpnet.org/professional_practice/best_practices/
Submission deadline: July 1, 2018
The objective of the Best Practice Award program is to encourage the development of innovative or creative pharmacy practice programs or innovative approaches to existing pharmacy practice challenges in health systems within the state of Illinois.
Applicants will be judged on their descriptions of programs and practices employed in their health system based on the following criteria:
- Innovativeness / originality
- Contribution to improving patient care
- Contribution to institution and pharmacy practice
- Scope of project
- Quality of submission
Eligibility
Applicants must be a member of ICHP for a minimum of 90 days prior to the submission deadline and practice in a health system setting. More than one program can be submitted by a health system for consideration.
Instructions for preparing manuscript
Each entry for the Best Practice Award must include a manuscript prepared as a Word document, double-spaced using Times New Roman 12-pitch type. A header with the paper title and page number should appear on each page. The manuscript should not exceed 2000 words in length (not counting references), plus no more than a total of 6 supplemental graphics (tables, graphs, pictures, etc.) that are relevant to the program. Each picture, graph, figure, and table should be mentioned in the text and prepared as a separate document clearly labeled.
The manuscript should be organized as a descriptive report using the following headings:
- Introduction, Purpose, and Goals of the program
- Description of the program
- Experience with and outcomes of the program
- Discussion of innovative aspects of programs and achievement of goals
- Conclusion
Format
Submissions will only be accepted via online submission form. The manuscript will be forwarded to a pre-defined set of reviewers. Please do not include the names of the authors or affiliations in the manuscript to preserve anonymity.
All applicants will be notified of their status within three weeks of the submission deadline. Should your program be chosen as the winner:
- The program will be featured at the ICHP Annual Meeting. You will need to prepare a poster to present your program and/or give a verbal presentation. Guidelines will be sent to the winner.
- You will be asked to electronically submit your manuscript to the ICHP KeePosted for publishing. This program will be accredited for CPE and will require that you complete material for ACPE accreditation.
- You will receive a complimentary registration to the ICHP Annual Meeting, recognition at the meeting and a monetary award of $1,000 distributed to your institution.
Non-winning submissions may also be considered for publication in the ICHP KeePosted, but your permission will be obtained beforehand.
If you have any questions related to the program please contact Trish Wegner at trishw@ichpnet.org.
Thank you to PharMEDium, Division of AmerisourceBergen for providing a grant for this year's Best Practice Award!
The 2016 & 2017 Best Practice Winners Available for CPE!
MALDI-TOF alone versus MALDI-TOF combined with real-time antimicrobial stewardship interventions on time to optimal therapy in patients with positive blood cultures
Authors: Maya Beganovic, PharmD and Sarah M. Wieczorkiewicz, PharmD, BCPS
Advocate Lutheran General Hospital, Park Ridge, Illinois
Implementation of Integrated Telepharmacy Services Achieve a Health-System Standard of Pharmacy Care
Authors: Sandra M. Salverson, PharmD, BCPS and Jerry Storm, RPh
OSF Healthcare System, Peoria, Illinois
Click here to learn how ICHP members can obtain free CPE credit.
Features
2018 Annual Meeting
Taking Pharmacy to New Heights
College Connection
Midwestern University Chicago College of Pharmacy
Legislative Day: Advocating Together
College Connection
by Kelly Moran, PS-2, ICHP Member

Attending the 12th Annual Pharmacy Legislative Day “Under the Dome” this March was not my first experience advocating for the pharmacy profession. I had the opportunity this past summer to meet with Illinois senators, representatives, and their staff on Capitol Hill. I remember feeling terrified in my white coat standing in front of the U.S. Capitol building about to meet with these powerful individuals. I felt so small. I then took a look around to see the other hundreds of student pharmacists standing alongside me and I felt how strong we were together. As I sat in the auditorium at Legislative Day this year, I had that same feeling of relief. As students, we have the power to make a difference whether it be through making phone calls, letter writing, attending events such as Legislative Day, or even just educating each other.
This year at Legislative Day we discussed the stance that we want to take as pharmacists on certain bills. A bill discussed that we were not supporting was HB5442 regarding the requirement of hospitals to report all controlled substances prescribed and administered to the Prescription Monitoring Program (PMP). The original purpose of the PMP is to identify patients “shopping” for controlled substances and obtaining large amounts of controlled substances. Based on my volunteer experience in a hospital, implementing this would be extremely difficult and a burden on hospital pharmacists. Also, it would be a burden for community pharmacists to have to search through unnecessary information in the PMP reported by inpatient pharmacists. A bill discussed of which we were supportive was HB5747 regarding the addition of prescribing and dispensing of hormonal contraceptives in the scope of pharmacy practice in Illinois. It is exciting to see this potential advancement for our profession. This bill is a perfect example of where pharmacy in Illinois is headed in the future.
For
the moment, advocating does not always feel worthwhile because there is no
automatic reward, outcome, or change in the profession. It takes years for
certain bills to pass and for our profession and to see a change in our own day
to day practice. It is not easy, but by attending these events and coming
together we have a voice and we are heard.
Roosevelt University College of Pharmacy
My Reflection as President of RUCOP-SSHP
College Connection
by Kimberly Zaleski, PS3, President Roosevelt University College of Pharmacy
As we transition our SSHP e-board positions to the Class of 2020, I wanted to take this opportunity to reflect on the great strides taken to successfully promote our organization at RUCOP. When I was elected President of our student chapter, I did not realize the opportunities of professional growth that would be available. Each event that we planned for this past school year was planned with professional growth in mind. Not only did our e-board members benefit from our events, but the presence of SSHP on campus has significantly grown. It was a very humbling experience to offer journal clubs and see other students learning from an article that was presented. After each journal club, it was easy to see that the students were becoming more comfortable with statistics and were able to interpret the results and conclusions of a study to make clinical recommendations.
In addition to journal clubs, we hosted multiple events so students could learn more about the residency process. Early in Fall term, we offered Residency 101 which gave a brief overview of what residency is and what the path to obtaining a residency looks like. Recently, we held the annual Residency Roundtable event. There was a great turnout of students and pharmacy residents from Northwestern Memorial Hospital and Advocate Christ Medical Center. During the event, there was plenty of thoughtful conversation and great questions. One of our major goals this year was to promote residency focused events so that our students can ultimately make the best decision for their future careers. We presented a poster at the Midyear clinical meeting on what P1 and P3 students knew about post-graduate opportunities. Our results indicated that some students knew what a residency was but the majority of students were unsure about post-graduate opportunities. Although we have not yet followed up with the Class of 2019 and 2020 with a new survey to see what they know now, my hope is based on the events hosted that more students have a better idea of what a residency has to offer.
In addition to these two events, we offered a CV workshop and brought in guest speakers to talk about initiatives and projects that they have completed during residency. It was a great opportunity to hear what the residents have done and how it has impacted their line of work. We also hosted events to also give back to our community including a blood drive through LifeSource on campus. We were able to help save 45 lives thanks to our blood donors. Members of SSHP and our student government organization also volunteered at a local food pantry to pack food for families that were in need.
Looking back on my year as the RUCOP student chapter SSHP President, I could not have asked for a better e-board to bring ideas to life. I am also beyond grateful for our faculty advisors that supported us throughout the year. I am excited to use what I have learned as an SSHP member to help me succeed during my APPE rotations and during my career as a pharmacist. Opportunities that were presented from ASHP and ICHP helped shape me as a leader and I hope that I can continue being involved in leadership positions throughout my career.
Rosalind Franklin University
ICHP P1 Liaison Reflection
College Connection
by Brit Der, PS-1 ICHP Liaison, Rosalind Franklin University of Medicine and Science, School of Pharmacy
Pharmacy school is difficult and requires a great deal of studying. Yet, I learned that earning the title of Doctor of Pharmacy is more than just studying. It also pertains to the various experiences and opportunities that you encounter. Adapting to my first year in pharmacy school was challenging. I also had the privilege of being appointed as the first year pharmacy student liaison for ICHP. Balancing this officer role made it quite an interesting year.
My role was to act as a communication resource between the chapter, the Dean, and the first year class. The opportunity to attend e-board meetings allowed me to observe the different responsibilities of each role, and the teamwork it required to prepare different events as well as meetings. As the communication resource, I was aware of all the different events that were held by the organization such as the Diabetes Expo and Legislative Day. All the events that I attended provided me with more insight in the clinical field of pharmacy and were great learning experiences. Personally, I am deciding upon the field of pharmacy which I want to pursue, but serving in ICHP has influenced me tremendously to consider the clinical setting.
In addition, serving as the P1 Liaison provided a positive outlet from the constant studying. The liaison position provided me with something different and exciting. The opportunity to connect with others was also an aspect that I did not expect to receive from the position. I had the opportunity to meet many upperclassmen and faculty in our school of pharmacy as well as even alumni that attended some of the events. I was able to slowly learn how to network and gradually come out of my comfort zone. I believe that this was one of the most important aspects that I learned. Mainly because knowing that the pharmacy network is fairly small, any individual in the field that I met was a potential important connection for my future. Overall, the P1 Liaison position was a rewarding experience. I learned a great deal about the clinical field of pharmacy, connected with many future colleagues, and ultimately learned how to manage an officer position with the workload of pharmacy school. I believe that this is a great officer role for incoming first year students that provides a great way to get involved as well as learn more about the clinical field of pharmacy. Studying is essential, but the learning experiences are what shape you as you earn the prestigious title Doctor of Pharmacy.
More
Welcome New Members!
|
New Member |
Recruiter |
|
William Clafshenkel |
Vicelle Sibal |
|
Meagan Conrath |
|
|
Lucas Dyer |
|
|
Tina Dyer |
|
|
Derek Holycross |
Jerry Storm |
|
Satoru Ito |
|
|
Tara Jebrael |
Claudia Muldoon |
|
Linhong Amy Long |
|
|
Jennifer Mayer |
|
|
Nicholas Moffett |
Vicelle Sibal |
|
Noha Mohamed |
|
|
Mogboluwaga Oginni |
|
|
Ravikumar Patel |
Vicelle Sibal |
|
Tatjana Petrova |
|
|
Zachary Ress |
|
|
Yvonne Rodriguez |
|
|
Danielle Sanchez |
|
|
Amanda Sek |
|
|
Karen Serafini |
Upcoming Events
AHA Heart Walk
Rockford Walk - 6/16/18
http://www2.heart.org/site/TR…
Oakbrook (Chicago) Walk - 9/22/18
http://www2.heart.org/site/TR…
Carterville Walk - 10/6/18
http://www2.heart.org/site/TR…
 Thursday, July 12, 2018 - NOON
Thursday, July 12, 2018 - NOON
Safe
Management of Drug Shortages
Natasha Nicol, PharmD, FASHP
Champions LIVE Webinar
Accredited for pharmacists and pharmacy technicians | 0.5
contact hour (.05 CEU)
- When: Saturday, August 25th, 2018
All day - Description: ICHP Student Leadership Retreat
Invitation Only
Location TBA
- When: September 13-15th, 2018
All day - Description: 2018 ICHP Annual Meeting
at Drury Lane Theatre and Conference Center,
Oakbrook Terrace, IL
Officers and Board of Directors
Executive Officers
 Travis Hunerdosse
Travis Hunerdosse
President
(312) 926-6124
 Charlene Hope
Charlene Hope
Immediate Past President
(708) 783-5933
chope@macneal.com
 Noelle Chapman
Noelle Chapman
President-Elect
 Kathryn Schultz
Kathryn Schultz
Treasurer
(312) 926-6961
kathryn_schultz@rush.edu
Regional Directors
 Amy Boblitt
Amy Boblitt
Regional Director Central
(217) 788-3015
boblitt.amy@mhsil.com
 Elise Wozniak
Elise Wozniak
Regional Director Northern
elise.m.wozniak@gmail.com
 Lynn Fromm
Lynn Fromm
Regional Director
Southern
(618) 391-5539
fromml@andersonhospital.org
Division Directors
 Mary Lee
Mary Lee
Organizational Affairs Director
(630) 515-7311
mleexx@midwestern.edu
 Karin Terry
Karin Terry
Professional Affairs Director
(309) 655-3390
Karin.l.terry@osfhealthcare.org
 Lara Ellinger
Lara Ellinger
Educational Affairs Director
(312) 926-3571
laelling@nm.org
 Carrie Vogler
Carrie Vogler
Marketing Affairs Director
(217) 545-5394
cvogler@siue.edu
Technician Representative
Network and Committee Chairs - non-voting
 Bernice Man
Bernice Man
Chairman, New Practitioners Network
(773) 702-9641
bernice.man.pharmd@gmail.com
 Abby Kahaleh
Abby Kahaleh
Ambulatory Care Network Chair
akahaleh@roosevelt.edu
 Jennifer Phillips
Jennifer Phillips
Editor & Chairman KeePosted
Committee Chair,
Nominations Committee
(630) 515-7167
jphillips@midwestern.edu
 Milena McLaughlin
Milena McLaughlin
Assistant Editor, KeePosted News Journal
(630) 515-7293
mmclau@midwestern.edu
Student Chapter Presidents
Ashley Shinnick - Chicago State University College of Pharmacy
Ashinnic@csu.edu
Shivek Kashyap - Midwestern University Chicago College of Pharmacy
skashyap28@midwestern.edu
Sara Koehnke - Roosevelt University College of Pharmacy
skoehnke@mail.roosevelt.edu
Aprille Banchoencharoensuk - Rosalind Franklin University
College of Pharmacy
aprille.banchoencharoensuk@my.rfums.org
Kaylee Poole - Southern Illinois University Edwardsville School of Pharmacy
kapoole@siue.edu
David Silva - University of Illinois at Chicago College of Pharmacy
dsilva8@uic.edu
HyeRim Whang Kong - University of Illinois at Chicago -
Rockford Campus College of Pharmacy
hwhang2@uic.edu
ICHP Affiliates
Northern Illinois Society (NISHP)
Erika Hellenbart
President
ehellen@uic.edu
Denise Kolanczyk
President-elect
Antoine Jenkins
Immediate Past President
(773) 821-2592
at-jenkins@csu.edu
David Martin
Treasurer
dwmartin0713@gmail.com
Milena McLaughin
Secretary
(630) 515-7293
mmclau@midwestern.edu
Vera Kalin
Technician Representative
West Central Society of Health-System Pharmacists (WSHP)
Ed Rainville
President
ed.c.rainville@osfhealthcare.org
Metro East Society (MESHP)
Jared Sheley
President
jpsheley@gmail.com
Sangamiss Society of Heath-System Pharmacists
Julie Downen
President
(217) 788-3953
downen.julie@mhsil.com
Billee Samples
President-elect
Katelyn Conklen
Immediate Past President
Conklen.Katelyn@mhsil.com
Vacant Roles at Affiliates; President, Rock Valley Society; Southern IL Society; Sugar Creek Society

 Jennifer Arnoldi
Jennifer Arnoldi Scott Meyers
Scott Meyers Christopher Crank
Christopher Crank Clara Gary
Clara Gary
