Official Newsjournal of the Illinois Council of Health-System Pharmacists

November 2021
Volume 47 Issue 4
Columns
Educational Affairs Meeting News
Professional Affairs - Call for Entries
Features
You Want Me to Talk to a Legislator???
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
More
Officers and Board of Directors
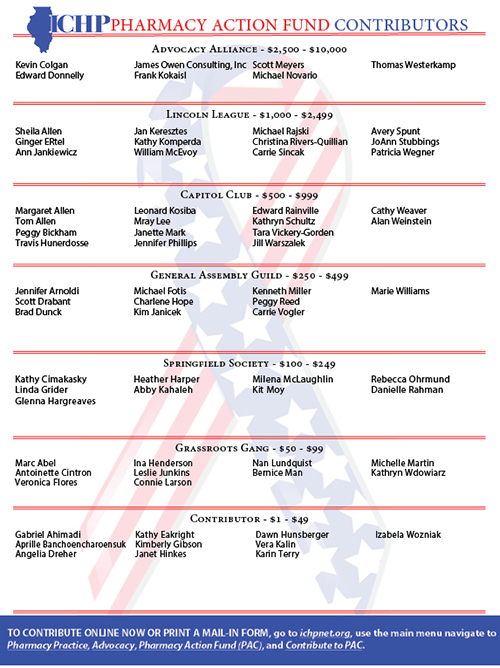
ICHP Pharmacy Action Fund (PAC) Contributors
KeePosted Info

Loves Park, IL 61111-8653
Phone: (815) 227-9292
Fax: (815) 227-9294
ichpnet.org
KeePosted
Official Newsjournal of the Illinois Council of Health-System Pharmacists
EDITOR
Jennifer Phillips
ASSISTANT EDITOR
Milena McLaughlin
MANAGING EDITOR
Scott Meyers
ASSISTANT MANAGING EDITOR
Trish Wegner
DESIGN EDITOR
Leann Nelson
EXECUTIVE VICE PRESIDENT
Scott Meyers
VICE PRESIDENT - PROFESSIONAL SERVICES
Trish Wegner
DIRECTOR OF OPERATIONS
Maggie Allen
INFORMATION SPECIALIST
Heidi Sunday
CUSTOMER SERVICE AND
PHARMACY TECH TOPICS™ SPECIALIST
Jo Ann Haley
ACCOUNTANTS
Jan Mark
COMMUNICATIONS MANAGER
Leann Nelson
ICHP Mission Statement
Advancing Excellence in Pharmacy
ICHP Vision Statement
ICHP dedicates itself to achieving a vision of pharmacy practice where:
- Pharmacists are universally recognized as health care professionals and essential providers of health care services.
- Patients are aware of the training, skills, and abilities of a pharmacist and the fundamental role that pharmacists play in optimizing medication therapy.
- Formally educated, appropriately trained, and PTCB certified pharmacy technicians manage the medication distribution process with appropriate pharmacist oversight.
- Pharmacists improve patient care and medication safety through the development of effective public policies by interacting and collaborating with patients, other health care professionals and their respective professional societies, government agencies, employers and other concerned parties.
- Evidence-based practices are used to achieve safe and effective medication therapies.
- There are an adequate number of qualified pharmacy leaders within the pharmacy profession.
- Pharmacists take primary responsibility for educating pharmacy technicians, pharmacy students, pharmacist peers, other health professionals, and patients about appropriate medication use.
KeePosted Vision
As an integral publication of the Illinois Council of Health-System Pharmacists, the KeePosted newsjournal will reflect its mission and goals. In conjunction with those goals, KeePosted will provide timely information that meets the changing professional and personal needs of Illinois pharmacists and technicians, and maintain high publication standards.
KeePosted is an official publication of, and is copyrighted by, the Illinois Council of Health-System Pharmacists (ICHP). KeePosted is published 10 times a year. ICHP members received KeePosted as a member benefit. All articles published herein represent the opinions of the authors and do not reflect the policy of the ICHP or the authors’ institutions unless specified. Advertising inquiries can be directed to ICHP office at the address listed above. Image disclaimer: The image used in the Pharmacy Tech Topics™ advertisement is the property of © 2017 Thinkstock, a division of Getty Images.
Copyright © 2018, Illinois Council of Health-System Pharmacists. All rights reserved.
 Directly Speaking
Directly Speaking
An Update from the Task Force
by Scott A. Meyers, Executive Vice President
The third open meeting (all of them are open by law) of the Collaborative Pharmaceutical Task Force has come and gone and unfortunately, we are still waiting for two of the eight voting members to be appointed. I’m not pointing fingers, Mr. Senate President Cullerton, but if the organized labor groups who concocted HB2392 last year to create this mess in the first place can’t get their stuff together to recommend an appropriate appointee to you for appointment, please appoint Garth Reynolds to represent IPhA and let us move on!
As I have mentioned in the past in other articles in previous issues of KeePosted, each of the four legislative leaders (Senate President, Senate Minority Leader, House Speaker and House Minority Leader) each had two appointments to make for this Task Force. House Minority Leader Jim Durkin, R- Burr Ridge made quick work of his appointments from ISMS (Scott Reimers) and ICHP (me). Senate Minority Leader Bill Brady, R-Bloomington followed the lead quickly and while House Speaker Madigan, D- Chicago, took his time and provided a twist by not appointing the Illinois Retail Merchants Association President, Rob Karr, as expected but got the job done before the second meeting.
The saddest part of this portion of the update is that during the three past meetings, organized labor hasn’t even bothered to send someone to at least sit in the audience and participate from there. The instigators don’t seem to be interested in solving the many identified problems any time soon. Unfortunately, nothing will happen until they get to the table and start participating.
As for the meetings, as reported previously, the first meeting was a review of the Task Force’s charge and initial identification of next steps. The second meeting or first real step was to hear an expert on “whistleblower” law talk about what is and what is not in the Pharmacy Practice Act already. The answer is pretty much nothing.
The March meeting (number 3) brought in staff from NABP to describe laws in other states that may address several of the 16 identified issues the task force is charged to review. There are some promising statutes and rules out there that may be useful but again, no steps can be taken until the task force is at full strength.
At the April Meeting of the task force, experts on e-prescribing will be present to discuss automated refills, e-discontinuation and other technology related topics. The meeting is set for April 10th at 1:30 pm in Chicago and Springfield. It will be open to the public should any interested party wish to participate. The specific locations have not yet been identified but will be posted on the IDFPR website prior to the meeting date.
The task force has had some good discussions and could be ready to tackle some of these issues in the next few meetings if and when the final two appointees are approved. I hope that we have a full task force for the next meeting and that we don’t have to repeat the discussions of the previous two. Garth Reynolds, IPhA Executive Director has been present at all three previous meetings and is expected to be appointed shortly. So we know he is ready to go. Let’s hope that organized labor gets their stuff together quickly so that we can move forward with the undertakings of the task force and improve care for our patients!
 President's Message
President's Message
Elevate Yourself - Keys to Learning New Skills
by Travis Hunerdosse, PharmD, MBA President, ICHP
The profession of pharmacy is ever changing to meet the needs of patients and the health care industry. As professionals, we need to equip ourselves with the knowledge and skills to meet these new demands. Elevating yourself through continuing professional development prepares you to reach your full potential in order to seize new opportunities in healthcare. You are ready for the challenge, but you may ask yourself where do I start?
People who are committed to life long learning are more likely to perform better at their job, find a new job or receive a promotion. Elevating yourself takes a commitment and developing habits to ensure you are always learning new skills. There are 4 habits that can influence your continuing learning of new skills.1
- Focus on emerging skills. The pharmacy and healthcare landscape is always changing and the requirements of technicians and pharmacists change with it. Learning the latest skills ensures that you stay relevant in your area of practice. Keep up with hot topics in pharmacy and emerging job opportunities. Once you identify a gap, take advantage of the opportunity to learn a new something new or broaden your expertise.
- Get synchronous. Learning on-line or by yourself may cause you to loose motivation and give up on learning. To avoid this, align yourself with others who share the same interest. Forming a study group for technician or pharmacist board certification is one way to stay motivated and ensure your goal is met. This interaction among the group fosters deeper learning and keeps everyone motivated.
- Implement learning immediately. Benefit your patients and your organization by acting on the new skills that you have acquired. This can be achieved by finding opportunities to use your new skills. Volunteer to serve on a task force, committee or lead a project.
- Set a golden benchmark. You need to have a clear goal in mind of what you want to achieve. It helps if you determine what you want to get out of your professional development plan. Developing a professional objective will help you stay focused as you learn. Learning is a life long process and your goals are likely to change over time as you achieve them and determine the next one.
Commit to Continuing Professional Development
Having served as the Director of Educational Affairs Division, I appreciate the importance of Continuing Professional Development for technicians and pharmacists. This can be achieved through various avenues such as on line or live continuing education (CE) activities, attending professional organization meetings like the ICHP Spring and Annual Meetings or ASHP Midyear and Summer Meetings, or obtaining advance certifications through the Pharmacy Technician Certification Board (PTCB) or the Board of Pharmacy Specialties (BPS).
Obtaining continuing education credits is only the first step of continuing professional development. In order to achieve the next level, you need to put new information or skills acquired into practice. In order to get started, focus on one or two educational programs that you enjoyed and learned something new. Perhaps it was a technician-focused session on USP 800 or a guideline update on the management of diabetes. The next step would be to learn more on the topic by finding related CE, journal articles and on-line resources. Once you are armed with the knowledge and skills, it is time to put it into practice by implementing changes or stepping up and volunteering for a committee or project.
Attending professional meetings is a great way to obtain live CE; however there are many more ways to do this. Take advantage of being around colleagues and exchanging new ideas. Some of the best innovations come from talking it through with a colleague who has a similar interest or has already implemented a successful change or program. After attending a particular session you may want to have further discussion with others who attended the same session to gain their insights and share ideas. Many professional meetings are also offering special interest networking sessions such as ambulatory practice, women in leadership, and medication safety just to name a few. This is a great opportunity to meet experts in the field and fosters great discussions.
Board certification is a great avenue for technicians and pharmacists to demonstrate their expertise in a particular area of pharmacy practice. The Pharmacy Technician Certification Board (PTCB) now offers an advanced certification for technicians in sterile compounding. Through this advanced certification technicians can learn new knowledge and skills that demonstrate expertise in safe sterile compounding. A commitment to developing advanced skills and qualifications benefits our health-systems and patients.
Another way to elevate yourself is through mentoring. Mentoring is a great way to keep your skills and knowledge sharp. In order to be an effective preceptor of student technicians and pharmacists, preceptors must keep up with the latest skills, regulations, or treatments in their area of expertise. In addition, students bring new information and ideas throughout the learning experience and push you to learn as well.
Elevating yourself can also come through presenting at a state or national meeting or publishing an article. Volunteering to give a presentation or publish an article pushes you to learn all you can about the subject that you will be presenting or writing about. It requires you to perform additional research and dig deeper into the topic so you are prepared to give it your best and address all potential questions that your audience may pose.
Let’s not forget to take advantage of opportunities outside of pharmacy or healthcare. All of us have interests outside of work such as playing sports or a musical instrument, gardening, or cooking. Enrolling in classes and participating in activities that are outside of the profession gives you a break from the day-to-day and equips you with new skills that you may not obtain otherwise. In addition, there are valuable lessons that can be translated back into your work. Don’t forget to take care of “you”, as this allows you to stay sharp, focused and at your peak so you can provide the very best care for patients.
Elevate yourself by developing habits to be a life long learner and commit to continuing professional development. Learning new skills elevates your practice and enables you to take the very best care of your patients.
Reference:
- Kehoe, M. 4 Habits of people who are always learning new skills. HBR. https://hbr.org/2018/01/4-habits-of-people-who-are-always-learning-new-skills January 31, 2018. Accessed March 8, 2018.

Columns
Board of Pharmacy Update
Highlights of the March Meeting
by Scott A. Meyers, Executive Vice President
The March 13th Board of Pharmacy Meeting was held at the Illinois Department of Financial and Professional Regulation Building at 320 West Washington in Springfield. These are the highlights of that meeting.
New Board Member Introduced – Board Chair, Yash Patel introduced Ryan McCann as a new pharmacist member of the Board of Pharmacy. Ryan will replace Ned Milenkovich who served over 10 years on the Board. Mr. Patel thanked Mr. Milenkovich for his many years of service to the State and its citizens.
NABP Annual Meeting – The National Association of Boards of Pharmacy will conduct its Annual Meeting in Denver on May 5-8, 2018. Yash Patel will serve as the Illinois Delegate to the meeting with Ned Milenkovich as first alternate and Denise Scarpelli as second alternate.
Department Update – Luciene Doler, Board of Pharmacy’s new general counsel replacing Katy Straub, provided news from the Department of Financial and Professional Affairs. The Collaborative Pharmaceutical Task Force has met twice so far with another meeting scheduled for that afternoon in Springfield and Chicago. There are still two task force members to be appointed representing IPhA and organized labor.
The public comments on the draft compounding rules are being reviewed by Department staff and a final draft is expected soon. ICHP will notify members of their details when they are approved and when they will become effective.
Robert Nelson and Janelle Kirby were present and introduced as pharmacy investigators.
Election of the Board Vice Chair– with the departure of Mr. Milenkovich, the Board elected Al Carter as its new Vice Chair. Mr. Carter will become the alternate delegate at the NABP Annual Meeting in May and Ms. Denise Scarpelli will become 2nd alternate.
Legislative Update – As ICHP Executive Vice President, I provided this month’s update. The Spring Session of the General Assembly is well underway and there are a variety of bills impacting pharmacy. Several bills address the opioid crisis by amending the Controlled Substance Act. These and all the bills ICHP is monitoring are discussed in the Government Affairs Report in this issue of KeePosted.
Next Meeting – The next meeting of the Board is set for May 15th to begin at 10:30 am in the James R. Thompson Center at Randolph and LaSalle in downtown Chicago. These meetings are open to the public and pharmacists, pharmacy technicians and pharmacy students are encouraged to attend.
Educational Affairs
New Oncology Drugs of 2017: Novel Intravenous Approvals
by Christopher Campbell, PharmD, BCPS, BCOP, Northwestern Memorial Hospital; Janna Afanasjeva, PharmD, BCPS, Northwestern Memorial Hospital; Chloe Majkowski, PharmD Candidate 2018, University of Illinois Chicago
With major advances in science and technology over the past few decades, the pharmaceutical industry has discovered better drug targets and applied this to drug development. Simultaneously, the Food and Drug Administration (FDA) introduced fast track, breakthrough therapy, and priority review designations, along with accelerated approval pathways, which have allowed new medications for treating serious conditions to reach the market much sooner than ever before. As Figure 1 demonstrates, expedited approvals for fast track, orphan drug, and accelerated approvals have increased to approximately 100, 40, and 10 respective designations or approvals per year.1 In 2017, 12 of 46 (26%) total novel drug approvals were in hematology and oncology alone, including 9 new molecular entities and 7 new monoclonal antibodies.2-4 The year 2017 also saw biologics license applications (BLAs) approved for the first 2 chimeric antigen receptor (CAR) T-Cell therapies, axicabtagene ciloleucel and tisagenlecleucel. These therapies are completely new methods of gene therapy utilizing modified versions of the patient’s own immune system to attack certain cancers in the blood. The purpose of this article is to provide a brief overview of the intravenous drug approvals in oncology for year 2017 that showcase innovation and the great strides being accomplished in treating cancer. Drug reviews include information on indications, special designations, efficacy, safety, and place in guidelines. Table 1 provides additional information on dosage forms, administration, and cost.
Avelumab (Bavencio®)
Indication
Avelumab received accelerated approval for metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients 12 years of age and older and for locally advanced or metastatic urothelial carcinoma (UC) in patients who have progressed on or after a platinum-containing chemotherapy regimen.5 The MCC indication had priority review, breakthrough therapy, and orphan drug status, as avelumab was the first FDA approved treatment for this disease. Urothelial carcinoma, a difficult to treat tumor, was given priority review.6,7 Avelumab inhibits programmed death-ligand 1 (PD-L1) receptor located on tumor cells, blocking the inhibition of T-cells and restoring immune response to tumor cells. Avelumab also engages the innate immune system and induces antibody-dependent cell-mediated cytotoxicity (ADCC).
Efficacy
The accelerated approvals of avelumab were based on 2 clinical trials: JAVELIN Merkel 200 and the UC cohort of the JAVELIN Solid Tumor trial.5 JAVELIN Merkel 200 was an open-label phase 2 study of avelumab monotherapy (10 mg/kg IV every 2 weeks) in 88 patients with metastatic MCC, who had prior chemotherapy.5,8 At the time of publication, the primary endpoint, the objective response rate (ORR), was 31.8% (95% confidence interval [CI], 21.9% to 43.1%) with 8 (9%) complete responses (CR) and 20 (23%) partial responses. Median progression-free survival (PFS) was 2.7 months, and median overall survival (OS) was 11.3 months. Results after 1 year follow-up included an updated ORR of 33%, due to 2 additional patients achieving a CR. The duration of response (DOR) in 29 patients was 86% over 6 months and 45% over 12 months. Responses were independent of PD-L1 tumor expression.
JAVELIN Solid Tumor was an open-label, single-arm, large phase 1b study of avelumab (10 mg/kg IV every 2 weeks) with a pooled analysis of 2 cohorts consisting of 241 patients with metastatic UC, in whom 95.4% had progressed on platinum-based therapy.5,9 At the time of abstract publication, there was a demonstrated primary endpoint ORR of 17.6% (95% CI, 12.0 to 24.6) in 27 out of 153 patients with at least 6 months follow-up. Median PFS was 6.4 weeks (95% CI, 6.1 to 11.4); however, median DOR was not reached. At the time of the FDA approval, ORR response dropped to 13.3%, and a median DOR had still not been reached (range in months of 1.4+ to 17.4+).5
Safety
In the JAVELIN Merkel 200 study, the most common adverse reactions reported were fatigue (50%), muscle pain (32%), diarrhea (23%), nausea (22%), infusion-related reaction (22%), rash (22%), decreased appetite (20%), and peripheral edema (20%).5 Immune-related adverse reactions included treatment-emergent abnormalities such as grade 3 to 4 elevated liver enzymes, increased bilirubin, and creatinine kinase elevation. Anemia was the most common reason for dose interruption. Permanent discontinuation was required for ileus, nephritis, transaminitis, and pericardial effusion. The UC cohort of the JAVELIN Solid Tumor trial also reported common infusion-related reactions (22.8%) and fatigue (12.0%).9 According to the published abstract, Grade 3 to 4 immune-related adverse reactions occurred in 11.6% of patients including 1 treatment-related death due to pneumonitis. However, the package insert describes that 14 patients (6%) from the pooled cohort died due to pneumonitis, respiratory failure, sepsis, cerebrovascular accident, or gastrointestinal adverse events.5
Guidelines
The NCCN Bladder Cancer guideline recommends avelumab in UC as a second-line single agent systemic therapy option for locally advanced or metastatic disease.10 The NCCN MCC guideline recommends avelumab for disseminated clinical M1 disease with or without surgery and/or radiation therapy.11
Axicabtagene ciloleucel (Yescarta™)
Indication
Axicabtagene ciloleucel is approved for use in adults with relapsed or refractory (r/r) B-cell lymphoma, such as diffuse large B-cell lymphoma (DLBCL) and other high grade lymphomas after fludarabine and cyclophosphamide lymphodepleting chemotherapy.12,13 Axicabtagene ciloleucel has not been studied in patients with primary central nervous system lymphoma and is not indicated for this population. The FDA granted priority review and orphan drug status to axicabtagene ciloleucel for this indication. Axicabtagene ciloleucel is the first treatment for B-cell lymphoma that targets CD19-expressing malignant cells with genetically modified autologous T-cells encoded with a CAR. Binding of CAR T-cells to CD19 activates a T-cell signaling cascade leading to the activation, proliferation, secretion of inflammatory cytokines, and ultimately to elimination of CD19 target cells.
Efficacy
Axicabtagene ciloleucel was approved for r/r B-cell lymphoma based on results from the ZUMA-1 trial.12 The ZUMA-1 trial was an open-label, phase 2, multicenter, single-arm trial assessing the safety and efficacy of a single infusion of axicabtagene ciloleucel therapy in 111 patients enrolled (101 treated) with r/r B-cell non-Hodgkin lymphomas (NHLs), primarily DLBCL.14 Patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to1 were included, and 77% of the patients had refractory disease to 2 or more lines of therapy including autologous stem cell transplant.15 The primary endpoint was an investigator-assessed ORR of 82% with ongoing responses in 44% of patients at 8.7 months.14 Tocilizumab and/or steroid use did not impact ORR. The median PFS was 5.9 months (95% CI, 3.4 to 9.8), while median OS was not reached. Manufacturing time for axicabtagene ciloleucel was on average 17 days with a 99% success rate.
Safety
The most common adverse reactions reported in the ZUMA-1 trial include cytokine release syndrome (CRS) (94%), fever (86%), hypotension (57%), encephalopathy (57%), tachycardia (57%), fatigue (46%), headache (45%), decreased appetite (44%), chills (40%), diarrhea (38%), and other very common (≥20%) adverse reactions such as febrile neutropenia, infections-pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias.14 The most common (≥ 10%) Grade 3 or higher reactions include febrile neutropenia, fever, CRS, encephalopathy, infections-pathogen unspecified, hypotension, hypoxia, and lung infections. Nearly all patients (>95%) experienced a Grade ≥3 adverse reaction - most frequently anemia, neutropenia, and febrile neutropenia. Axicabtagene ciloleucel carries a boxed warning for CRS and neurologic events as Grade 3 or higher events were observed in 13% and 31% of patients, respectively.12 Axicabtagene ciloleucel is only available through a Risk Evaluation and Mitigation Strategies (REMS) to mitigate the risks of CRS and neurological toxicities.
Guidelines
The NCCN B-Cell Lymphomas guideline recommends axicabtagene ciloleucel as subsequent therapy after second-line therapy for DLBCL, primary mediastinal large B-cell lymphoma or double hit lymphoma.16 Axicabtagene ciloleucel is also recommended as subsequent therapy for patients with histologic transformation to DLBCL after treatment with ≥2 chemoimmunotherapy regimens, which included at least 1 anthracycline or anthracenedione-based regimen, unless contraindicated.
Copanlisib (Aliqopa™)
Indication
Copanlisib received accelerated approval for the treatment of relapsed follicular lymphoma (FL) in patients who have received 2 prior systemic therapies.17,18 The FDA granted orphan drug and fast track designation for copanlisib in the harder to treat third-line setting due to efficacy and safety profiles. Copanlisib’s mechanism of action is novel in that it inhibits mainly phosphatidylinositol-3-kinase (PI3K) alpha and delta isoforms expressed in malignant B-cells. This process causes apoptosis and inhibition of proliferation of malignant B-cell lines. Idelalisib, a first generation PI3K inhibitor, only inhibits a single PI3K-delta isoform.19
Efficacy
The results contributing to the efficacy and safety of copanlisib are only available from the manufacturer and have not been published yet. The approval of copanlisib is based on CHRONOS-1 trial, which was a single-arm, multicenter, phase 2 clinical trial.18 The trial enrolled 142 patients, which included 104 patients with follicular B-cell NHL who have received at least 2 therapies before. All patients were treated with at least rituximab and an alkylating agent in the past. The majority of patients (130/142) received 60 mg of copanlisib on days 1, 8, and 15 of a 28-day cycle. The ORR was 59% (95% CI, 49% to 68%). This medication received accelerated approval based on ORR, which is contingent on the results of a confirmatory trial.
Safety
The most common adverse reactions reported with copanlisib were hyperglycemia (54%), diarrhea (36%), decrease in energy and strength (36%), hypertension (35%), leukopenia (36%), neutropenia, thrombocytopenia (22%), nausea (26%), and lower respiratory tract infections (21%).18 Pneumonia (8%), pneumonitis (5%), and hyperglycemia (5%) were the most frequent serious adverse reactions. Side effects may be different from idelalisib as copanlisib is administered intravenously and bypasses the gastrointestinal (GI) tract in an attempt to minimize severe GI side effects such as colitis. Per the prescribing information, caution is advised for GI toxicity, pulmonary toxicity, hyperglycemia, hypertension, dermatologic toxicity, bone marrow suppression, and infection.
Guidelines
Per NCCN guidelines for B-cell lymphomas, copanlisib is listed as a second-line and subsequent therapy in patients with FL who are refractory to at least 2 prior therapies.16
Durvalumab (Imfinzi®)
Indication
Durvalumab received accelerated approval for the use in patients with locally advanced or metastatic UC who have experienced disease progression with platinum-containing chemotherapy.20,21 The FDA granted priority review and breakthrough therapy designations to durvalumab, and the agent was approved only days before the approval of avelumab, which can be also used in UC. Durvalumab is a monoclonal antibody that inhibits the interaction of PD-L1 with PD-1 and CD80. This mechanism differs from PD-1 inhibitors, as it acts at the ligand on tumor cells. This inhibition leads to the activation of T-cells and decrease in tumor size by allowing T-cells to kill tumor cells. Unlike avelumab, durvalumab is engineered to prevent ADCC. The FDA simultaneously approved the VENTANA PD-L1 assay to test for PD-L1 expression, which may predict the likelihood of response to durvalumab.22
Efficacy
The approval of durvalumab was based on a phase 1/2 open label study.21,23 The study enrolled 191 patients with locally advanced or metastatic UC who have progressed on or refused chemotherapy. Patients received durvalumab 10 mg/kg IV every 2 weeks for up to 12 months, until disease progression, or until unacceptable toxicity. The median follow-up time was 5.78 months and ranged from 0.4 to 25.9 months. The primary endpoint, the ORR, was 17.8% (95% CI, 12.7% to 24%). In patients with high PD-L1 expression, the ORR was 27.6% (95% CI, 19% to 37.5%). The ORR was 5.1% (95% CI, 1.4% to 12.5%) in patients with low or no expression of PD-L1. The median PFS was 1.5 months (95% CI, 1.4 to 1.9 months) with 2.1 months (95% CI, 1.4 to 2.8 months) in patients with high PD-L1 expression and 1.4 months (95%CI, 1.3 to 1.5 months) in patients with low or no PD-L1 expression. The median OS was 18.2 months (95% CI, 8.1 months to not estimable) with 20 months (95% CI, 11.6 to not estimable) in patients with high PD-L1 expression and 8.1 months (95% CI, 3.1 to not estimable) in patients with low or no PD-L1 expression.
Safety
In the clinical study for UC, Grade 3/4 adverse events occurred in 6.8% of patients.23 The most common adverse events were fatigue (19.4%), decrease in appetite (9.4%), diarrhea (8.4%), and rash (7.3%). Immune-mediated warnings and concerns apply, such as adrenal insufficiency, rash, type 1 diabetes, colitis, hepatitis, hypophysitis, pneumonitis, nephritis, and others known to be associated with cancer immunotherapy are listed in the prescribing information.
Guidelines
Per NCCN Bladder Cancer guideline, durvalumab is an option as a subsequent systemic therapy for locally advanced or metastatic bladder cancer after first-line therapy.10
Inotuzumab ozogamicin (Besponsa™)
Indication
Inotuzumab ozogamicin is approved for treatment of adult patients with r/r B-cell precursor acute lymphoblastic leukemia (ALL) which is associated with poor prognosis in adults.24,25 The FDA granted priority review, breakthrough therapy and orphan drug status for this indication associated with poor prognosis. Inotuzumab ozogamicin, an antibody-drug conjugate (ADC) for CD22, selectively binds to tumor cells expressing CD22.25 An internalization of ADC-CD22 complex by cells leads to release of N-acetyl-gamma-calicheamicin dimethylhydrazide, which contributes to double-strand DNA breaks, cell cycle arrest, and apoptosis.
Efficacy
The approval of inotuzumab ozogamicin was based on INO-VATE ALL study.25 This was an open-label, multicenter, randomized, phase 3 study of inotuzumab ozogamicin versus standard of care in 326 adult patients with r/r B-cell ALL (CD22-positive, Philadelphia chromosome (Ph)-positive or negative) requiring first or second salvage treatment.26 The administered dose was 0.8 mg/m2 (or 0.5 mg/m2 if CR or CR with incomplete hematologic response (CRi) was achieved after cycle 1) on day 1, 0.5 mg/m2 on day 8 and 15 of 21 to 28-day cycles. The standard of care consisted of fludarabine with cytarabine and granulocyte-colony stimulating factor, mitoxantrone with cytarabine, or high-dose cytarabine. The primary endpoints were CR and OS. The study showed a higher CR rate in 80.7% (95% CI, 72.1 to 87.7) of patients in the inotuzumab ozogamicin group versus 29.4% (95% CI, 21.0 to 38.8) of patients in the standard therapy group (p<0.001). The PFS was greater at 5 months in the inotuzumab ozogamicin group versus 1.8 months in the standard therapy group with a hazard ratio (HR) of 0.45 (97.5% CI, 0.34 to 0.61; p<0.001). The median OS was 7.7 months in the inotuzumab ozogamicin group versus 6.7 months in the standard therapy group (HR, 0.77; 97.5% CI, 0.58 to 1.03; p=0.04).
Safety
According to the INO-VATE ALL study, the most common non-hematologic adverse event in the inotuzumab ozogamicin was a veno-occlusive liver disease (11% in the inotuzumab ozogamicin group versus 1% in the standard therapy group).26 Common treatment emergent adverse events with inotuzumab ozogamicin consisted of nausea (31%), headache (28%), and pyrexia (32%). The boxed warning for the agent mentions veno-occlusive liver disease and a higher non-relapse mortality after hematopoietic stem cell transplant.25
Guidelines
Per the NCCN ALL guideline, inotuzumab ozogamicin is an option for patients with Ph-positive ALL who are refractory or intolerant to tyrosine kinase inhibitors (TKIs).27 This agent is also recommended in patients with r/r Ph-negative ALL. The guideline also states that inotuzumab ozogamicin is associated with hepatic problems and has an increased risk for post-hematopoietic stem cell transplant non-relapse mortality.
Tisagenlecleucel (Kymriah™)
Indication
Tisagenlecleucel was the first-in-class CAR T-cell therapy.28 Tisagenlecleucel is approved for use following fludarabine and cyclophosphamide lymphodepleting chemotherapy in patients up to 25 years of age with r/r B-cell precursor ALL.29 Tisagenlecleucel targets CD19-expressing malignant cells with genetically modified autologous T-cells encoded with a CAR. Binding of CAR T-cells to CD19 promotes T-cell signaling, expansion, activation, and persistence to eliminate target cells.
Efficacy
Tisagenlecleucel was approved for r/r B-cell ALL based on results from the ELIANA trial.29 The ELIANA trial was an open-label, multicenter, single-arm, phase 2 trial assessing the safety and efficacy of at least 1 treatment of tisagenlecleucel therapy (0.2 to 5.0 x 10^6 cells/kg for patients ≤50 kg or 1.0 to 2.5 x 10^8 cells/kg for patients >50 kg) in 88 enrolled (68 treated) pediatric and young adult patients (median age of 12 years) with r/r B-cell ALL.30 The primary endpoint was overall remission rate of 82.5% with duration of remissions in 64% of patients at 9 and 12 months. The relapse-free probability at 6 months remission onset was 75% (95% CI, 57% to 87%) and OS was not reported. Tisagenlecleucel cellular productmanufacturing failures prevented 7 (8.0%) patients from being treated.
Safety
The most common adverse reactions in the ELIANA trial include CRS (79%), hypogammaglobulinemia (43%), infections (41%), fever (40%), decreased appetite (37%), headache (37%), encephalopathy (34%), hypotension (31%), bleeding (31%), tachycardia (26%), nausea (26%), diarrhea (26%), vomiting (26%), viral infections (26%), hypoxia (24%), fatigue (22%), acute kidney injury (22%), and delirium (21%).30 Half of all patients with CRS in this trial required tocilizumab. The most common (≥ 10%) Grade 3 or higher reactions include CRS, febrile neutropenia, hypoxia, hypotension, increased liver enzymes, decreased appetite, hypokalemia, cytopenias, and neurological events. Two deaths occurred within 30 days of tisagenlecleucel treatment (one due to ALL progression during CRS and another due to cerebral hemorrhage with resolving CRS). Tisagenlecleucel carries a boxed warning for CRS and neurologic events as 49% and 18% of patients experience Grade 3 to 4 effects, respectively. There is a REMS in effect to mitigate the risks of CRS and neurological toxicities.
Guidelines
The NCCN ALL guidelines recommend tisagenlecleucel as a single-agent therapy for patients under 26 years old with r/r B-cell ALL after 2 or more relapses, and if Ph -positive B-cell ALL then also after failure of 2 TKIs.27
Table 1. Properties of newly approved IV oncology agents in 2017.5,12,18,21,25,29,31
Drug Name (Brand) |
Dosage Form(s) |
Dosing and Administration |
Costa |
Avelumab (Bavencio®) |
Single-dose vial; 200 mg/10 mL (20 mg/mL) preservative-free solution for injection |
10 mg/kg IV infusion over 60 minutes every 2 weeks |
$1,832/vial |
Axicabtagene ciloleucel (Yescarta™) |
Single-dose frozen suspension; variable number of cultured, genetically modified cells based on patient-specific weight |
Target dose of 2.0x106 to a maximum of 2.0x108 CAR-positive viable T-cells per kg as IV infusion after completing lymphodepleting chemotherapy on the fifth, fourth, and third day prior to treatment. |
$373,000/autologous cellular treatmentb |
Copanlisib (Aliqopa™) |
Single-dose vial; 60 mg lyophilized powder |
60 mg IV infusion over 60 minutes on days 1, 8, 15 every 28 day-cycle |
$5,040/vial |
Durvalumab (Imfinzi®) |
Single-dose vials; 120 mg/2.4 mL (50 mg/mL) and 500 mg/10 mL (50 mg/mL) solution for injection |
10 mg/kg IV infusion over 60 minutes once every 2 weeks |
120 mg/2.4 ml vial - $1,002/vial 500 mg/10 ml vial - $4,174.57/vial a |
Inotuzumab ozogamicin (Besponsa™) |
Single-dose vial; 0.9 mg lyophilized powder |
IV infusion over 60 minutes on days 1 (0.8 mg/m2), 8 (0.5 mg/m2), and 15 (0.5 mg/m2) of the first 21-day cycle; for subsequent cycles, the dose is 0.5 mg/m2 on day 1 if patients achieved CR or CRi or 0.8 mg/m2 in patients who have not achieved CR or CRi. The doses for day 8 and day 15 are the same as in the first cycle with the exception of subsequent cycles being 28 days long |
$22,440/vial |
Tisagenlecleucel (Kymriah) |
Single-dose frozen suspension; variable number of cultured, genetically modified cells based on patient-specific weight |
0.2 -5.0 x 106 CAR-positive viable T-cells per kg (patients 50 kg and below) 0.1-2.5 x 108 cells per kg (patients above 50 kg) as one infusion 2 to 14 days after completing lymphodepleting chemotherapy |
$475,000/autologous cellular infusionb |
aPricing is based on average wholesale price.
bPricing is based on manufacturer statement.
Abbreviations: CR = complete response; CRi = complete response with incomplete hematologic recovery; IV = intravenous.
Conclusion
This article reviewed 6 novel intravenous or cellular therapies approved in 2017 for at-need patient populations in malignancies such as those with MCC, UC, r/r B-cell lymphoma, ALL, and FL. This represents 33% of all new oncology medications, medication combinations, or biosimilars approved, and 13% of new hematology or oncology indications approved in 2017. As a condition of the accelerated approvals, ongoing clinical trials to confirm clinical benefits of avelumab, copanlisib, and durvalumab are needed. Overall these are much welcomed approvals, but come with significant costs. While exciting breakthroughs in cellular technology such as CAR T-Cell therapy can potentially increase survival, they carry a significant price tag, hospital admissions, and the need for ancillary high cost medications such as tocilizumab. It is yet to be seen how these costs will be weighed against other potential therapies, including stem cell transplants. With combined increases in the complexity of newly approved medications, sky-rocketed price tags, and expedited approval pathways, it has never been more important to advance the conversation that correlates cost with defined value for patients and healthcare systems.
References:
- Number of fast track designation requests granted. Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/fdatrack/view/track.cfm?program=cder&status=public&id=CDER-RRDS-Number-of-Fast-Track-Designations-granted&fy=2012. Updated September 30, 2017. Accessed January 14, 2018.
- CY 2017 CDER breakthrough therapy calendar year approvals. Food and Drug Administration website. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/
HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/
NDAandBLAApprovalReports/UCM494010.pdf. Updated December 31, 2017. Accessed January 14, 2018. - Drugs@FDA: FDA approved drug products. Food and Drug Administration website. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed January 14, 2018.
- Novel drug approvals for 2017. Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm537040.htm. Updated January 12, 2018. Accessed January 15, 2018.
- Bavencio [package insert]. Rockland, MA: EMD Serono, Inc; 2017.
- FDA grants accelerated approval to avelumab for urothelial carcinoma. Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm557162.htm. Updated May 9, 2017. Accessed January 15, 2018.
- Avelumab (Bavencio). Food and Drug Administration website. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm547965.htm. Updated May 30, 2017. Accessed January 15, 2018.
- Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385.
- Patel M, Ellerton J, Infante J, et al. Avelumab in patients with metastatic urothelial carcinoma: pooled results from two cohorts of the phase 1b JAVELIN solid tumor trial. Poster presented at ASCO 2017. J Clin Oncol. 35:6; suppl 330.
- NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer Version 1.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Published January 8, 2018. Accessed January 8, 2018.
- NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma Version 1.2018. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. Published September 18, 2017. Accessed January 8, 2018.
- Yescarta [package insert]. Santa Monica, CA: Kite Pharma, Inc; 2017.
- FDA approves axicabtagene ciloleucel for large B-cell lymphoma. Food and Drug Administration website. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm581296.htm. Updated October 25, 2017. Accessed January 15, 2018.
- Locke FL, Neelapu SS, Bartlett NL, et al. Clinical and biological covariates of outcomes in ZUMA-1: a pivotal trial of axicabtagene ciloleucel (Axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (r-NHL). Presented at: American Society for Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL.
- A phase 1-2 multi-center study evaluating axicabtagene ciloleucel in subjects with refractory aggressive non-hodgkin lymphoma (ZUMA-1). Clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT02348216. Updated December 17, 2017. Accessed February 8, 2018.
- NCCN Clinical Practice Guidelines in Oncology. B-Cell Lymphomas Version 7.2017. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Published December 5, 2017. Accessed January 7, 2017.
- FDA grants accelerated approval to copanlisib for relapsed follicular lymphoma. Food and Drug Administration website. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm576098.htm. Updated September 14, 2017. Accessed January 15, 2018.
- Aliqopa [package insert]. Whippany, NJ: Bayer; 2017.
- Zydelig [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2017.
- Durvalumab (Imfinzi). Food and Drug Administration website. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555930.htm. Updated May 1, 2017. Accessed January 15, 2018.
- Imfinzi [package insert]. Wilmington, DE: AstraZeneca; 2017.
- Roche receives FDA approval for complementary PD-L1 (SP263) biomarker test in urothelial carcinoma. Ventana website. http://www.ventana.com/roche-receives-fda-approval-complementary-pd-l1-sp263-biomarker-test-urothelial-carcinoma/. Updated May 2, 2017. Accessed February 12, 2018.
- Powles T, O'Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411.
- FDA approves inotuzumab ozogamicin for relapsed or refractory B-cell precursor ALL. Food and Drug Administration website. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm572133.htm. Updated August 17, 2017. Accessed January 15, 2018.
- Besponsa [package insert]. Philadelphia, PA: Pfizer; 2017.
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740-753.
- NCCN Clinical Practice Guidelines in Oncology. Acute Lymphoblastic Leukemia Version 5.2017. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf. Published October 27, 2017. Accessed January 8, 2018. In.
- FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. Food and Drug Administration website. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm574154.htm. Updated September 7, 2017. Accessed January 25, 2018.
- Kymriah [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017.
- Buechner J, Grupp S, Maude S, et al. Global registration trial of efficacy and safety of CTL019 in pediatric and young adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL): update to the interim analysis. Clin Lymphoma Myeloma Leuk. 17:S263-S264.
- LexiComp [database]. Hudson, OH: Wolters Kluwer Health; 2018. https://online.lexi.com/lco/action/home. Accessed January 5, 2018.

Educational Affairs Meeting News
ICHP 2018 Spring Meeting - Get the Handouts & the App!
|
||||||||||||||||
Government Affairs Report
The Fun Has Begun!
by Jim Owen and Scott Meyers
Bill introduction is complete and now the real work begins for ICHP’s Government Affairs Division and the staff. With 3083 new bills this spring and nearly 5800 bills from last year that could be resurrected to sift through, the 2018 Spring Legislative Session is proving to be more excitement than we would like. Especially with the on-going deliberations of the Collaborative Pharmaceutical Task Force we discussed in February well underway!
As you will see from the attached list of proposed legislation, there is no shortage of pharmacy and health care related propositions to monitor! We will discuss a few briefly and then recommend you review the entire list for yourselves to see if one we didn’t mention grabs your personal attention!
SB2524 Amends the Environmental Protection Act to create the Pharmaceutical Disposal Task Force. The task force will be responsible for coordinating a public information campaign on the benefits of properly disposing of unused pharmaceutical products. The bill identifies task force members including one pharmacist and one representative of the retail sector (most likely from the Illinois Retail Merchants Association – IRMA). The sponsor of the bill is Sen. Chapin Rose – Champaign, R.
SB2529 Amends various acts prohibiting Illinois licensing authorities from denying, not renewing, suspending or revoking professional licenses as a result of default on state guaranteed student loans or scholarships. The sponsor of the bill is Sen. Steve Stadelman – Rockford, D.
SB2849 Creates the Prescription Drug Repository Program Act and requires the Department of Public Health to establish a prescription drug repository where citizens may donate unused medications for redistribution to the indigent. Participation in the program by pharmacies is voluntary and pharmacies who do participate in the program may charge a handling fee to fill prescriptions but may not charge for the medications. In addition, civil and criminal liability protection is provided for participants in the program unless harm is caused by willful and wanton behaviors. Sponsor of the bill is Sen. Patrician Van Pelt – Chicago, D.
SB2952 and HB4907 Amends the Controlled Substance Act by allowing the Department of Human Services to adopt rules allowing pharmacists and physicians who have registered to access the PMP to authorize licensed and non-licensed individuals who have been properly trained with regard to HIPAA requirements to access the PMP on their behalf. The bill also adds one dentist to the PMP Advisory Committee’s Peer Review Subcommittee. Sponsors of the bills are Sen. Melinda Bush – Grayslake, D and Rep. Mike McAuliffe – Chicago, R.
SB3431 Amends the Controlled Substance Act to restrict first prescriptions of opiates to a 7-day supply for patients 18 and older and all prescriptions for opiates for patients under 18 to a 7-day supply. There are exceptions for cancer or palliative care patients. The sponsor of the bill is Sen. Sue Rezin – Peru, D.
HB4650 Amends the Controlled Substance Act to allow pharmacists who are employed by Managed Care Organizations and Pharmacy Benefit Managers to access the PMP. Sponsored by Rep. Mike Zalewski – Riverside, D.
HB4707 Creates the Prescription Drug Task Force Act and establishes a 17 member task force to examine the extent of over prescribing of opioids has on patients and to make recommendations for future legislation to address the opioid crisis in Illinois. Sponsor of the bill is Rep. Sue Scherer – Decatur, D.
HB5442 Amends the Controlled Substance Act to require hospitals to transmit all controlled substance doses administered to inpatients to the PMP by the end of the business day. Requires all pharmacies to report by the end of the business day instead of the next business day. Sponsor of the bill is Rep. Jim Durkin – Burr Ridge, R.
HB5747 Amends the Pharmacy Practice Act to allow pharmacists to prescribe and dispense hormonal contraceptives (tablets, patch or ring) after completing approved training. Requires the pharmacist to provide the patient with a self-assessment form that will be reviewed and evaluated by the pharmacist prior to prescribing and dispensing. For patients 18 years of age and older or patients under 18 years of age if they have previously received a prescription for one of these products. Sponsor of the bill is Rep. Michelle Mussman – Schaumburg, D.
Here are all the bills ICHP is currently monitoring. If you have suggestions or an interest in these bills, please contact Scott Meyers at scottm@ichpnet.org.
2018 Illinois General Assembly Bill Summary
Bill Number |
Sponsor |
Summary |
Location |
ICHP Position |
SB0316 |
Steans – Chicago, D |
Amends the Illinois Clinical Laboratory and Blood Bank Act. Makes a technical change in a Section concerning the short title. Cannabis taxation – 21 or older buy cannabis be taxed like alcohol purchase |
In Assignments |
|
SB0336 Sam001 |
Harmon - Oak Park, D |
Amends SB336 by replacing everything after the enacting clause with the following: “Section 1. This Act may be referred to as the Alternatives to Opioids Act of 2018. Section 5. the Compassionate Use of Medical Cannabis Pilot Program Act is amended by changing sections 5, 10, 60, and 160 as follows…” Sam001 supporting cannabis as MAT for opioid use disorder |
Placed on Calendar Order of 3rd Reading February 27th 2018 2/22/2018 Added co-sponsor Daniel Biss 2/20/2018 |
|
SB1888 & HB3479 |
McCann – Jacksonville, R Feigenholtz – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. In addition to other specified actions required under the Code, requires a managed care community network that contracts with the Department of Healthcare and Family Services to establish, maintain, and provide a fair and reasonable reimbursement rate to pharmacy providers for pharmaceutical services, prescription drugs and drug products, and pharmacy or pharmacist-provided services. Provides that the reimbursement methodology shall not be less than the current reimbursement rate utilized by the Department for prescription and pharmacy or pharmacist-provided services and shall not be below the actual acquisition cost of the pharmacy provider. Requires a managed care community network to ensure that the pharmacy formulary used by the managed care community network and its contract providers is no more restrictive than the Department's pharmaceutical program. Effective July 1, 2018. |
Assignments in Senate Rules in House |
|
SB2189 |
Connelly – Naperville, R |
Creates the Medicaid Smart Card Pilot Program Act. Requires the Director of the Department of Healthcare and Family Services to establish a Medicaid Smart Card Pilot Program to reduce the total amount of expenditures under the State's Medical Assistance Program. Provides that the pilot program shall be designed to reduce the average monthly cost under the State's Medical Assistance Program for recipients within the pilot program area by an amount that is at least sufficient to recover the cost of implementing the pilot program. Provides that the Director shall determine the geographic area to be included in the pilot program and may contract with an independent entity for the purpose of developing and implementing the pilot program. Contains provisions on required activities under the pilot program, including the distribution of Medicaid Smart Cards to designated recipients; measures the Department might take to implement the pilot program; annual evaluations; reporting requirements; extension or expansion of the pilot program; the confidentiality of health information; reports to the Inspector General; and rulemaking authority. |
To Subcommittee on Special Issues (HS) 2/21/2018 |
Neutral |
SB2226 |
Nybo - Lombard, R |
Amends the State Police Act. Provides that a physician, physician's assistant with prescriptive authority, or advanced practice registered nurse with prescriptive authority who provides a standing order or prescription for epinephrine auto-injectors in the name of the Department of State Police shall incur no civil or professional liability, except for willful and wanton conduct, as a result of any injury or death arising from the use of an epinephrine auto-injector. Amends the Illinois Police Training Act. Provides that a physician, physician's assistant with prescriptive authority, or advanced practice registered nurse with prescriptive authority who provides a standing order or prescription for epinephrine auto-injectors in the name of a local governmental agency shall incur no civil or professional liability, except for willful and wanton conduct, as a result of any injury or death arising from the use of an epinephrine auto-injector. Makes conforming changes to the Medical Practice Act of 1987 and the Public Health Standing Orders Act. Effective immediately. |
First Reading Referred to Rules Committee in the House 2/27/2018 Chief House Sponsor Deb Conroy 2/21/2018 |
|
SB2334 |
Murphy - Elk Grove Village, D |
Amends the University of Illinois Hospital Act and Hospital Licensing Act. Provides that a hospital shall maintain a metal detector at each point of entry into the hospital. Provides that a hospital shall ensure that all members of the public, other than the employees of the hospital who display proper credentials, who enter the hospital at a point of entry are subjected to screening by a metal detector. Provides that individuals subject to screening shall include, but not be limited to, individuals in wheelchairs. Defines "point of entry". Effective July 1, 2018. |
Postponed - Public Health 2/27/2018 |
Neutral |
SB2465 |
Mulroe- Chicago, D |
Amends the Illinois Public Aid Code. Makes a technical change in a Section concerning medical services. |
Referred to Assignments 1/30/2018 |
|
SB2524 |
Rose - Champaign, R |
Sen. Amendment 1: Amends the Environmental Protection Act by creating the Pharmaceutical Disposal Task Force. The task force will coordinate a statewide public information campaign to highlight the benefits and opportunities of properly disposing of pharmaceutical products. Identifies the members of the task force and responsibilities. |
Senate Committee Amendment No. 1 Filed, Referred to Assignments 3/9/18 |
Neutral |
SB2529 |
Stadelman - Rockford, D |
Amends various acts to remove provisions allowing or requiring licensing authorities to deny, not renew, suspend, or revoke professional licenses for defaulting on an educational loan or scholarship provided by or guaranteed by a State agency. Effective immediately. |
Postponed - Licensed Activities and Pensions 2/15/2018 |
Support |
SB2827 HB2511 |
Murphy - Elk Grove Village, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. Provides that drugs prescribed to residents of the following facilities are not subject to prior approval as a result of the 4-prescription limit: long-term care facilities as defined in the Nursing Home Care Act; community-integrated living arrangements as defined in the Community-Integrated Living Arrangements Licensure and Certification Act; and supportive living facilities as defined in the Code. |
Assigned to Public Health 3/01/18 |
Neutral |
SB2849 |
Van Pelt - Chicago, D |
Creates the Prescription Drug Repository Program Act. Requires the Department of Public Health to, by rule, establish a prescription drug repository program, under which any person may donate a prescription drug or supplies needed to administer a prescription drug for use by an individual who meets eligibility criteria specified by the Department. Sets forth requirements that prescription drugs or supplies must meet in order to be accepted and dispensed under the program. Provides that no drugs or supplies donated under the prescription drug repository program may be resold. Provides that nothing in the Act requires that a pharmacy or pharmacist participate in the prescription drug repository program. Provides for civil and criminal immunity for drug and supply manufacturers and individuals in relation to the donation, acceptance, or dispensing of prescription drugs or supplies under the prescription drug repository program. Imposes conditions on any rulemaking authority. Amends the Pharmacy Practice Act, the Wholesale Drug Distribution Licensing Act, the Senior Pharmaceutical Assistance Act, the Illinois Food, Drug and Cosmetic Act, the Illinois Controlled Substances Act, and the Cannabis and Controlled Substances Tort Claims Act to provide that persons engaged in donating or accepting, or packaging, repackaging, or labeling, prescription drugs to the extent permitted or required under the Prescription Drug Repository Program Act are exempt from provisions of those other Acts that might prohibit or otherwise regulate such activity. |
Assigned to Judiciary 2/21/18 |
Neutral |
SB2889 |
Rose – Champaign, R |
Creates the Epinephrine Administration Act. Provides that a health care practitioner may prescribe epinephrine pre-filled syringes in the name of an authorized entity where allergens capable of causing anaphylaxis may be present. Provides that an authorized entity may acquire and stock a supply of undesignated epinephrine pre-filled syringes provided the undesignated epinephrine pre-filled syringes are stored in a specified location. Requires each employee, agent, or other individual of the authorized entity to complete a specified training program before using a pre-filled syringe to administer epinephrine. Provides that a trained employee, agent, or other individual of the authorized entity may either provide or administer an epinephrine pre-filled syringe to a person whom the employee, agent, or other individual believes in good faith is experiencing anaphylaxis. Provides that training under the Act shall be valid for 2 years. Requires the Department of Public Health to approve training programs, to list the approved programs on the Department's website, and to include links to training providers' websites on the Department's website. Contains provisions concerning costs, limitations, and rulemaking. Defines terms. Amends the School Code. In provisions concerning epinephrine administration, provides that epinephrine may be administered with a pre-filled syringe. Makes conforming changes. |
Senate Committee Amendment No. 1 Referred to Assignment 3/02/2018 |
|
SB2952 HB4907 |
Bush – Grayslake, D |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately |
Placed on Calendar Order of 2nd Reading Feb 28th, 2018 Do Pass Public Health; 009-000-000 2/27/18 |
|
SB3015 |
Koehler - Peoria, D |
Amends the School Code. With regard to the self-administration and self-carry of asthma medication, provides that a school district, public school, charter school, or nonpublic school may authorize a school nurse or trained personnel to (i) provide undesignated asthma medication to a student for self-administration only or to any personnel authorized under a student's Individual Health Care Action Plan or asthma action plan, plan pursuant to Section 504 of the federal Rehabilitation Act of 1973, or individualized education program plan to administer to the student that meets the student's prescription on file, (ii) administer an undesignated asthma medication that meets the prescription on file to any student who has an Individual Health Care Action Plan or asthma action plan, plan pursuant to Section 504 of the federal Rehabilitation Act of 1973, or individualized education program plan that authorizes the use of asthma medication; and (iii) administer an undesignated asthma medication to any person that the school nurse or trained personnel believes in good faith is having respiratory distress; defines "undesignated asthma medication" and "respiratory distress". Changes the definition of "asthma medication" to mean quick-relief asthma medication that is approved by the United States Food and Drug Administration for the treatment of respiratory distress. Provides that a school nurse or trained personnel may administer undesignated asthma medication to any person whom the school nurse or trained personnel in good faith believes to be experiencing respiratory distress (i) while in school, (ii) while at a school-sponsored activity, (iii) while under the supervision of school personnel, or (iv) before or after normal school activities. Provides that a school district, public school, charter school, or nonpublic school may maintain a supply of an asthma medication in any secure location where a person is most at risk. Provides that a training curriculum to recognize and respond to respiratory distress may be conducted online or in person. Specifies training requirements. Makes other changes. Effective immediately. |
Senate Committee Amendment NO.1 Referred to Assignments 3/7/18 Added Chief Co-Sponsor 2/27/2018 |
|
SB3116 |
Hunter – Chicago, D |
Amends the Nurse Practice Act. In provisions concerning written collaborative agreements, restores the ability of podiatric physicians to collaborate with advanced practice registered nurses. Makes other changes. Effective immediately. |
Assigned to Licensed Activities and Pensions 2/27/18 |
|
SB3170 |
Stadelman - Rockford, D |
Amends the Pharmacy Practice Act and the Illinois Food, Drug and Cosmetic Act. Provides that a prescription for medication other than controlled substances shall be valid for up to 15 months from the date issued for the purpose of refills, unless the prescription states otherwise. |
Assigned to Licensed Activities and Pensions 2/27/18 |
Oppose |
SB3431 |
Rezin - Peru, R |
Amends the Illinois Controlled Substances Act. Provides that when issuing a prescription for an opiate to a patient 18 years of age or older for outpatient use for the first time, a practitioner may not issue a prescription for more than a 7-day supply. Provides that a practitioner may not issue an opiate prescription to a person under 18 years of age for more than a 7-day supply at any time and shall discuss with the parent or guardian of the person under 18 years of age the risks associated with opiate use and the reasons why the prescription is necessary. Provides that notwithstanding this provision, if, in the professional medical judgment of a practitioner, more than a 7-day supply of an opiate is required to treat the patient's acute medical condition or is necessary for the treatment of chronic pain management, pain associated with a cancer diagnoses, or for palliative care, then the practitioner may issue a prescription for the quantity needed to treat that acute medical condition, chronic pain, pain associated with a cancer diagnosis, or pain experienced while the patient is in palliative care. Provides that the condition triggering the prescription of an opiate for more than a 7-day supply shall be documented in the patient's medical record and the practitioner shall indicate that a non-opiate alternative was not appropriate to address the medical condition. Provides that these provisions do not apply to medications designed for the treatment of substance abuse or opioid dependence. Effective immediately. |
Assigned to Public Health 2/27/18 |
Neutral |
SB3498 |
Sims, Jr. -Chicago, D |
Amends the Managed Care Reform and Patient Rights Act. Requires a policy or plan sponsor to notify the prescribing physician and the patient in writing 60 days before making a formulary change that alters the terms of coverage or discontinues coverage for a prescribed drug that the patient is receiving. Contains provisions for receiving the notice electronically. Provides that a policy or plan sponsor may provide the patient with the written notification, along with a 60-day supply of the prescription drug, at the time the patient requests a refill. Provides that nothing in the provisions prohibits insurers or pharmacy benefit managers from using certain managed pharmacy care tools so long as an exception process is in place allowing the prescriber to petition for coverage a non-preferred drug if sufficient clinical reasons justify an exception to the normal protocol. |
Assigned to Insurance 2/27/18 |
Neutral |
HB4096 |
Harris – Chicago, D |
Amends the Medical Assistance Article of the Illinois Public Aid Code. Provides that the Department of Healthcare and Family Services shall require each Medicaid Managed Care Organization to list as preferred on the Medicaid Managed Care Organization's preferred drug list every pharmaceutical that is listed as preferred on the Department's preferred drug list. Provides that the Department shall not prohibit, or adopt any rules or policies that prohibit, a Medicaid Managed Care Organization from: (i) covering additional pharmaceuticals that are not listed on the Department's preferred drug list; or (ii) removing from the Medicaid Managed Care Organization's preferred drug list any prior approval requirements applicable under the Department's preferred drug list. Provides that the Department shall not require a Medicaid Managed Care Organization to utilize a single, statewide preferred drug list and shall not prohibit a plan from negotiating drug pricing concessions or rebates on any drug with pharmaceutical companies, unless otherwise required by federal law. Provides that no later than July 1, 2018, the Department shall develop a standardized format for all Medicaid Managed Care Organization preferred drug lists in cooperation with Medicaid Managed Care Organizations and stakeholders, including, but not limited to, community-based organizations, providers, and individuals or entities with expertise in drug formulary development. Requires each Medicaid Managed Care Organization to post its preferred drug list on its website without restricting access to enrolled members and to update the preferred drug list posted on its website within 2 business days of making any changes to the preferred drug list, including, but not limited to, any and all changes to requirements for prior approval. Effective immediately. |
Placed on Calendar Order of 3rd Reading - Short Debate Approved for Consideration Rules Committee 1/30/2018 Added Co-Sponsor 3/8/18 |
|
HB4166 |
Harris – Chicago, D |
Creates the Health Insurance Claims Assessment Act. Imposes an assessment of 1% on claims paid by a health insurance carrier or third-party administrator. Provides that the moneys received and collected under the Act shall be deposited into the Healthcare Provider Relief Fund and used solely for the purpose of funding Medicaid services provided under the medical assistance programs administered by the Department of Healthcare and Family Services. |
Assigned to Appropriations-Human Services Committee 1/24/2018 |
|
HB4270 |
Olsen - Downers Grove, R |
Amends the Good Samaritan Act. Provides that a free medical clinic shall not be liable for civil damages as a result of acts or omissions in providing medical treatment, diagnosis, or advice, except for willful or wanton misconduct. |
To Tort Liability Law Subcommittee 3/7/18 |
|
HB4331 |
Conner – Romeoville, D |
Amends the Counties Code. Provides that in every case in which an opioid overdose is determined to be a contributing factor in a death, the coroner shall report the death and the age, gender, race, and county of residence, if known, of the decedent to the Department of Public Health. Amends the University of Illinois Hospital Act and the Hospital Licensing Act. Requires every hospital to report the age, gender, race, and county of residence, if known, of each patient diagnosed as having an opioid overdose to the Department within 48 hours of the diagnosis. Amends the Department of Public Health Powers and Duties Law of the Civil Administrative Code of Illinois. Requires the Department to adopt rules to implement the reporting requirements. Requires the Department to annually report to the General Assembly the data collected. |
Placed on Calendar 2nd Reading- Short 3/8/2018 |
|
HB4436 |
Chapa LaVia -Aurora, D |
Amends the Mental Health and Developmental Disabilities Code. Provides that notwithstanding any of the provisions of the Code concerning the administration of psychotropic medication and electroconvulsive therapy, psychotropic medication or electroconvulsive therapy may be administered pursuant to a power of attorney for health care under the Powers of Attorney for Health Care Law or a declaration for mental health treatment under the Mental Health Treatment Preference Declaration Act over the objection of the recipient if the recipient has not revoked the power of attorney or declaration for mental health treatment as provided in the relevant statute. Effective immediately. |
Referred to Rules 1/31/2018 Added Chief Co-Sponsor 2/13/2018 |
|
HB4440 |
Gabel - Evanston, D |
Amends the Nursing Home Care Act. Provides that the Department of Public Health shall provide facilities with educational information on all vaccines recommended by the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices, including, but not limited to, the risks associated with shingles and how to protect oneself against the varicella-zoster virus. Requires a facility to distribute the information to each resident who requests the information and each newly admitted resident. Allows the facility to distribute the information to residents electronically. Effective January 1, 2019. |
Arrived in Senate Placed on Calendar Order of First Reading 3/9/2018 Added Chief Co-Sponsor 3/9/2018 |
|
HB4479 |
Cabello – Rockford, R |
Amends the Criminal Code of 2012 relating to first degree murder. Adds and eliminates aggravating factors for which the death penalty may be imposed. Amends the Code of Criminal Procedure of 1963. Eliminates provision that abolishes the sentence of death. Enacts the Capital Crimes Litigation Act of 2018. Provides that all unobligated and unexpended moneys remaining in the Death Penalty Abolition Fund on the effective date of the amendatory Act shall be transferred into the Capital Litigation Trust Fund. Amends the State Appellate Defender Act. Provides that in cases in which a death sentence is an authorized disposition, the State Appellate Defender shall provide trial counsel with legal assistance and the assistance of expert witnesses, investigators, and mitigation specialists from funds appropriated to the State Appellate Defender specifically for that purpose by the General Assembly. Provides that the Office of State Appellate Defender shall not be appointed to serve as trial counsel in capital cases. |
Referred to Rules Committee 2/2/2018 Added Chief Co-Sponsor 2/28/18 |
|
HB4571 |
Ammons - Champaign, D |
Amends the Cannabis Control Act. Makes a technical change in a Section concerning the short title. |
Referred to Rules Committee 2/6/18 |
|
HB4643 |
Burke - Chicago, D |
Amends the Illinois Physical Therapy Act. Provides that the limitation on determining a differential diagnosis shall not in any manner limit a physical therapist from establishing a relevant diagnosis. In the definition of "documented current and relevant diagnosis" and in provisions concerning disciplinary actions, removes language requiring a diagnosis to be substantiated by a physician, dentist, advanced practice registered nurse, physician assistant, or podiatric physician. Effective immediately. |
Assigned to Health Care Licenses Committee 3/5/18 Added Chief Co-Sponsor 3/1/2018 |
|
HB4650 |
Zalewski - Riverside, D |
Amends the Illinois Controlled Substance Act. In a provision allowing pharmacists to authorize a designee to consult the Prescription Monitoring Program on their behalf, defines "pharmacist" to include, but be not limited to, a pharmacist associated with a health maintenance organization or a Medicaid managed care entity providing services under the Illinois Public Aid Code. Effective immediately. |
Assigned to Health Care Licenses Committee 2/21/2018 |
Oppose |
HB4696 |
Olsen - Downers Grove, R |
Amends the Use Tax Act, the Service Use Tax Act, the Service Occupation Tax Act, and the Retailers' Occupation Tax Act. Provides that, beginning on January 1, 2019, the tax is not imposed on prescription medicines or prescription drugs. Effective immediately. |
Referred to Rules Committee 2/13/18 |
|
HB4699 |
Carroll - Buffalo Grove, D |
Amends the Good Samaritan Act. Provides that a person who in good faith and without fee administers epinephrine to another person who is suffering from a severe allergic reaction shall not be liable for civil damages as a result of his or her acts or omissions, except for willful or wanton misconduct on the part of the person in administering the epinephrine. |
Referred to Rules Committee 2/13/18 |
|
HB4707 House Amendment 001 |
Scherer - Decatur, D |
House Amendment 001: Creates the Prescription Drug Task Force Act and a Prescription drug task Force with 17 members, one member from ICHP that will study the extent of over prescribing of opioids to patients and to make recommendations for future legislation to address the issue. |
Placed on Calendar 2nd Reading – Short Debate 3/8/2018 |
Neutral |
HB4900 |
Guzzardi - Chicago, D |
Creates the Illinois Generic Drug Pricing Fairness Act. Provides that a manufacturer or wholesale drug distributor shall not engage in price gouging in the sale of an essential off-patent or generic drug. Provides that the Director of Healthcare and Family Services or Director of Central Management Services may notify the Attorney General of any increase in the price of any essential off-patent or generic drug under the Medical Assistance Program under the Illinois Public Aid Code or a State health plan, respectively, that amounts to price gouging. Provides that whenever the Attorney General has reason to believe that a manufacturer or wholesale drug distributor of an essential off-patent or generic drug has violated the Act, the Attorney General shall send a notice to the manufacturer or wholesale drug distributor requesting a specified statement. Provides that within 45 days after receipt of the request, the manufacturer or wholesale drug distributor shall submit the statement to the Attorney General. Provides that to accomplish the objectives and carry out the duties prescribed in the Act, the Attorney General may issue subpoenas or examine under oath any person to determine whether a manufacturer or wholesale drug distributor has violated the Act. Provides that upon petition of the Attorney General, a circuit court may issue specified orders against violations of the Act. Contains provisions concerning the disclosure of financial information provided by a manufacturer or wholesale drug distributor to the Attorney General. Effective January 1, 2019. |
State Mandates Fiscal Note Filed 3/8/2018 Added Co-Sponsor 3/7/2018 |
|
HB4906 |
McAuliffe – Chicago, R |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately. |
Referred to Rules Committee 2/14/2018 |
|
HB4907 SB2952 |
McAuliffe - Chicago, R |
Amends the Illinois Controlled Substances Act. Provides that the Department of Human Services, in consultation with the Advisory Committee, shall adopt rules allowing licensed prescribers or pharmacists who have registered to access the Prescription Monitoring Program to authorize a licensed or non-licensed designee (rather than any designee) employed in that licensed prescriber's office or licensed pharmacist's pharmacy and who has received training in the federal Health Insurance Portability and Accountability Act to consult the Prescription Monitoring Program on their behalf. Requires the Clinical Director of the Prescription Monitoring Program to select 6 members (rather than 5 members), 3 physicians, 2 pharmacists, and one dentist, of the Prescription Monitoring Program Advisory Committee to serve as members of the peer review subcommittee. Effective immediately |
Assigned to Human Services Committee 3/5/18 Added Co-Sponsor 3/6/2018 |
|
HB4950 |
Feigenholtz – Chicago, D |
Creates the Early Mental Health and Addictions Treatment Act. Requires the Department of Healthcare and Family Services, and other specified agencies and entities, to develop a pilot program under which a qualifying adolescent or young adult may receive community-based mental health treatment from a youth-focused community support team for early treatment that is specifically tailored to the needs of youth and young adults in the early stages of a serious emotional disturbance or serious mental illness. Requires the Department to apply, no later than September 30, 2019, for any necessary federal waiver or State Plan amendment to implement the pilot program. Requires the Department to implement the pilot program no later than December 31, 2019 if federal approval is not necessary. Contains provisions concerning the creation of a community-based treatment model under the pilot program; the development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Requires the Department to develop an Assertive Engagement and Community-Based Clinical Treatment Pilot Program for individuals with opioid and other drug addictions. Contains provisions on in-office, in-home, and in-community services provided under the pilot program; application for a federal waiver or State Plan amendment to implement the pilot program; development of a pay-for-performance payment model; Department rules to implement the pilot program; and analytics and outcomes report. Effective immediately. |
Referred to Rules Committee 2/14/2018 Added Chief Co-Sponsor 3/6/2018 |
|
HB5442 |
Durkin - Burr Ridge, R |
Amends the Open Meetings Act. Provides that, for the purposes of the Act, "public body" does not include a Metropolitan Enforcement Group (MEG) Policy Board or drug task force composed or created by any combination of local law enforcement agencies. Amends the Criminal Code of 2012. Provides that a person commits drug-induced homicide when he or she violates delivery of a controlled substance or methamphetamine or a similar law of another jurisdiction, by unlawfully delivering a controlled substance to another, and the injection, inhalation, absorption, or ingestion of any amount of that controlled substance is a contributing cause of the person's death. Amends the Illinois Controlled Substances Act. Provides that controlled substances which are lawfully administered in hospitals or institutions licensed under the Hospital Licensing Act shall be reported under (rather than, exempt from) specified reporting provisions under the Act, and the prescription for the controlled substances ordered and the quantity actually administered (rather than, the reporting requirement only applies for more than a 72-hour supply of a discharge medication to be consumed outside of the hospital or institution). Provides that the information required to be transmitted under the prescription monitoring program must be transmitted not later than the end of the business day on which a controlled substance is dispensed, or at such other time as may be required by the Department of Human Services by administrative rule (rather than, at the end of the next business day on which the controlled substance is dispensed). |
Assigned to Judiciary – Criminal Committee 3/5/18 |
Oppose |
HB5602 |
Carroll - Buffalo Grove, D |
Amends the Safe Pharmaceutical Disposal Act. Provides that "unused medication" means any unopened, expired, or excess medication that has been dispensed for patient or resident care and that is in a liquid or solid form (rather than in a solid form). Makes related changes. |
Referred to Rules Committee 2/16/18 |
|
HB5747 |
Mussman - Schaumburg, D |
Amends the Pharmacy Practice Act. Provides that "practice of pharmacy" includes the prescribing and dispensing of hormonal contraceptive patches and self-administered oral hormonal contraceptives. Defines "hormonal contraceptive patch" as a transdermal patch applied to the skin of a patient, by the patient or by a practitioner, that releases a drug composed of a combination of hormones that is approved by the United States Food and Drug Administration to prevent pregnancy and "self-administered oral hormonal contraceptive" as a drug composed of a combination of hormones that is approved by the United States Food and Drug Administration to prevent pregnancy and that the patient to whom the drug is prescribed may take orally. Allows pharmacists to prescribe and dispense contraceptives to a person over 18 years of age and a person under 18 years of age only if the person has evidence of a previous prescription from a primary care or a women's health care practitioner. Requires the Department of Financial and Professional Regulation to adopt rules to establish standard procedures for pharmacists to prescribe contraceptives. Provides requirements for the rules to be adopted by the Department. Provides that all State and federal laws governing insurance coverage of contraceptive drugs and products shall apply to the provisions. |
Referred to Rules Committee 2/16/18 Added Co-Sponsor Reps 3/8/19 |
Support |
Senate Deadlines |
House Deadlines |
||
Bill Introduction |
February 16, 2018 |
February 16, 2018 |
Bill Introduction |
Senate bills out of Committee |
April 13, 2018 |
April 13, 2018 |
House Bills out of Committee |
Third Reading for Senate Bills |
April 27, 2018 |
April 27, 2018 |
Third Reading House Bills |
House Bills out of Committee |
May 11, 2018 |
May 18, 2018 |
Senate Bills out of Committee |
Third Reading House Bills |
May 25, 2018 |
May 25, 2018 |
Third Reading Senate Bills |
Adjournment |
May 31, 2018 |
May 31, 2018 |
Adjournment |
ICHPeople


Pharmacy Legislative Day 2018 was a great sucess!
Leadership Profile
Meet Bernice Man, PharmD
 My story: Bernice Man, PharmD
My story: Bernice Man, PharmD
Leadership position in ICHP: Chair of New Practitioners Network
Practice site: Practice Coordinator at Northwestern Medicine Specialty Pharmacy
I made a difference:
One my best experiences was with a recent interaction with an ulcerative colitis patient who expressed to me her appreciation for my work and reassurance in helping get her biologic medication covered.
Why pharmacy is for me:
My career path to pharmacy has been different than many other pharmacists as I previously worked in more arts-oriented fields such as acting, graphic design, and independent film. I joined pharmacy at an opportune time when the nature of our profession is in positive flux with the push for provider status and the emphasis on practicing at the top of our license. I truly enjoy having the opportunity to actively participate in making change in our profession through organizations such as ICHP. Through my work as the gastroenterology pharmacist in the specialty pharmacy, I also truly enjoy the opportunity to positively impact patients’ health and quality of life.
ICHP is great because:
ICHP is great because of the plethora of opportunities available to collaborate with like-minded people who actively participate in the progression of our profession.
First time joining ICHP:
I initially joined ICHP because of the SSHP booth at my pharmacy school’s student organization fair. At that time, I worked at a suburban community hospital as an inpatient pharmacy technician and already had in mind that I wanted to complete a residency. I had goals of increasing my involvement in SSHP so I subsequently ran for P1 Liaison. My SSHP leadership and ICHP involvement developed and grew from that point onward.
Special thanks for making me who I am today in my career:
There are a number of exceptional individuals who have inspired and guided me in my career thus far. I’d like to take this opportunity to thank Diana Isaacs, Bryan McCarthy, Katherine Miller, Jennifer Tryon, and Kevin Colgan. All of these individuals had and continue to have a strong impact and positive influence on my growth within pharmacy. I will always be so thankful and appreciative of their support.
Advice for a student:
I would advise student pharmacists to remain open-minded as one never knows where your life will lead, within and outside of pharmacy.
My special interests or hobbies outside of work:
Watching films (would highly recommend Phantom Thread and Loving Vincent) and going to concerts of many different genres, from classical to pop to new wave. I also enjoy playing mahjong with my family and going to baseball games.
Favorite restaurant or food:
I love restaurants and cuisines of all types, which is one of the best benefits of living in Chicago!
New Practitioners Network
Is There a Better Way to do That? From Practitioner to Resident - A Step Back to Look Forward
by Elana Nelson, PharmD, BCPS, PGY2 Drug Information Resident, University of Illinois - Chicago
It was over two years ago that I decided to pursue a non-traditional path in pharmacy. Although I enjoyed what I was doing at the time, I still had some regrets about not following what I loved after my PGY1 residency. I was encouraged when I received some advice from an older, experienced pharmacist. “Follow your passion”, he advised. He stated that he wished he could have done things differently many years ago when he was young and it was early in his career. I learned from him that fear is a huge deterrent. Fear is the main thing that can stop many people from pursuing their dreams. However, when you face your fears head-on, you can do unbelievable things. I decided to face my fears and applied for a PGY2 residency four years after completing my PGY1 residency. I have learned many valuable lessons from taking that step.
It was during the first month of my residency that I quickly realized I was not alone. Although my path in pharmacy is not traditional, there are many others who did not pursue a second year of residency immediately after completing a PGY1. Two of my preceptors completed a PGY2 residency several years after working as clinical pharmacists. These preceptors had a substantial impact on my development. They were there to provide support and inspiration because of their similar experiences.
In addition to these preceptors, I am thankful that I found a good mentor who has guided me through the more challenging aspects of residency training. The importance of having a mentor cannot be overemphasized. Much of my personal and professional success can be attributed to the advice I received from her. She has changed my perspective, helped me stay motivated, and continues to serve as a great resource.
What makes my PGY2 residency worthwhile is that I am doing the work I enjoy. It is true that a residency requires wearing many different hats and acquiring several skills simultaneously, but the bottom line is that I have found my passion. This makes the work not so hard.
Finally, the most important lesson I have learned is that sometimes our careers may lead to new experiences and opportunities that we might not have imagined. Keep yourself prepared for the unexpected experiences that may come your way. A pharmacy career is a journey that offers real opportunities, potential, and variety. It is one thing to be good at something, but to enjoy it and be very passionate about it is quite different.
When you find what you love, follow that passion. You will be more successful and happier if you do.
Professional Affairs
Pharmacist Burnout: A Decline in the Health of Healthcare Professionals
by Jen Phillips, PharmD, BCPS, FCCP, FASHP Associate Professor, Pharmacy Practice, Midwestern University Chicago College of Pharmacy and Jane Lee, PharmD Candidate, Midwestern University, Chicago College of Pharmacy
The Issue
The term “burnout” describes the characteristics of chronic, unrelieved stress that can result in running out of the sustenance necessary to continue functioning at a healthy capacity. Common signs involve emotional exhaustion, amplified depersonalization, and feelings of inadequacy in the workplace.1,2 Perhaps the most surprising of these three for a healthcare professional, is depersonalization, which involves viewing people as objects rather than human beings.2 Exhaustion and inefficacy may be reversible with appropriate rest and refocus, but depersonalization can result in a permanent psychological shift in the way healthcare providers think about their profession. A 2014 study analyzing the prevalence of burnout among practicing physicians (N=35,922) found that more than half (54.4%) exhibited at least one symptom of burnout.2 Moreover, these results represented a 10% increase in burnout compared to a similar study performed 3 years earlier in 2011.1
The Data
The consequences of clinician burnout include detrimental effects on quality, safety, patient satisfaction, and healthcare costs. Specifically, stress levels, burnout, and emotional exhaustion among physicians and nurses has been correlated with increased number of malpractice lawsuits,3 health-care associated infections,4 and mortality.5 From a patient perspective, depersonalization has been shown to affect patient satisfaction,6,7,8,9 and patient adherence to medical advice.10 These consequences substantiate a need for increased awareness of burnout and systematic changes to prevent it from occurring in all healthcare practitioners and trainees.
Most of the published research describing the prevalence of stress, depression, and other detrimental effects of burnout has involved the medical and nursing disciplines. Medical residents and students, in particular, have been found to have worse outcomes than age-similar individuals in different careers.11 Limited data exists for other members of the health care team, but there seems a high likelihood that these results can be extrapolated to other members of the healthcare team, including pharmacists.
One cross-sectional study evaluated the effects of job demands and interpersonal interactions of 566 hospital pharmacists on work-related outcomes, such as organizational and professional commitment, job satisfaction, burnout, and professional identify.12 The researchers based their objective on the job demands-resources model (JD-R). The JD-R model assumes that pharmacist burnout occurs through exhaustion and job disengagement from lack of resources.12 The researchers found a correlation between poor work environments (defined as high-demand/unpleasant interactions) and the frequency (r 2 =0.08, p<0.01) and intensity (r2 =0.07, p<0.01) of emotional exhaustion.12 In addition, there was also a correlation between positive work environments (defined as low-demand/pleasant encounters) and the frequency (r2 =0.11, p<0.001) and intensity (r2 =0.05, p<0.001) of feeling personal accomplishment.12 The results of this study support the JD-R model and provided an alternative method of looking at the quality of pharmacy practice work environments.
The impact of stress and burnout among trainees has also been evaluated. One study investigated stress levels in pharmacy residents.13 This cross-sectional study assessed stress reported by PGY-1 and PGY-2 residents through survey questionnaires sent to program directors from 2011 to 2012.13 To measure stress and negative affect levels, the 10-item Perceived Stress Scale (PSS10) and the Multiple Affect Adjective Checklist-Revised (MAACL-R) were utilized. Both are validated or widely used psychological tools. Age and program year did not result in significantly different PSS10 scores.13 However, MAACL-R scores for hostility were higher for PGY2 residents compared to PGY1 residents (50.83 vs. 48.62, respectively, p=0.017).13 Working more than 60 hours per week correlated with higher stress levels on the PSS10 (r=0.143, p=0.001) and higher scores on the MAACL-R hostility subscale (r=0.0088, p<0.05), depression subscale (r=0.118, p<0.05), and dysphoria subscale (r=0.119, p<0.05) for the entire sample13 This is important to note, as the data suggests that even with the American Society of Health-System Pharmacists’ (ASHP) current residency duty hour limitation set at a maximum of 80 hours per week, residents may still be experiencing inappropriate amounts of stress.
Strategies to Address the Issue
As a response to the data that continues to emerge on the consequences of healthcare worker burnout, many organizations have begun studying the issues further. The National Academy of Medicine (NAM) issued an Action Collaborative on Clinical Well-Being and Resilience in 2017.14 The collaborative consists of over 50 sponsoring organizations that have committed to addressing the issue of clinician burnout. The NAM document separates factors affecting healthcare professional well-being into individual and external categories.14 Sub-factors such as the healthcare role, relationships and social support, learned skills and abilities, and personal elements, are grouped into the individual category.14 The external category is composed of the work environment, regulatory environment, organizational elements, and society itself.14 Some factors – such as organizational element, learned skills and abilities, and relationships/social support - may require less time and resources for improvement. All of these elements have communication-based cores. Improving communication can assist with these three factors. Other factors, including the work or regulatory environment, may take more time and resources to change.
In an article published by the Annals of Family Medicine, the authors present reasons to expand the “Triple Aim” to the “Quadruple Aim”.15 The Triple Aim was a framework developed by the Institute for Healthcare Improvement (IHI), as an initiative to “enhance patient experience, improve population health, and reduce costs”.15 Improving the lives of “health care providers, including clinicians and staff” was the goal of the fourth addendum.15 Even though the data presented supporting this addendum was on nurse and physician burnout, the authors summarized that “those who deliver care” are the focus of the fourth aim.
To respond to the issue of clinician burnout, pharmacy and other healthcare organizations have begun developing and implementing policies advocating clinician well-being and resilience in the workplace. The American Medical Association (AMA) recognizes burnout in students, residents, and physicians, and has policies in place supporting collaboration with other groups to maintain continued awareness of this topic. They also encourage research, monitoring of the issue, and mindfulness education. For nurses, the American Nursing Association (ANA) has a separate policy regarding fatigue, safety, and health, to be upheld by both nurses and employers. The American Pharmacists Association (APhA) encourages employers to provide tools for stress and conflict management. Regarding working conditions on public safety, APhA opposes having a quota for prescriptions that need to be dispensed during a certain time period. ASHP has policies addressing several topics related to clinician burnout, including: workplace violence, intimidating or disruptive behaviors, support for second victims, and the relationship between staff fatigue and medication errors. In addition, ASHP is one of the sponsors of the National Academy of Medicine’s Action Collaborative on Clinician Well-Being and Resilience and is actively working toward developing policies and strategies to address the issue of clinician burnout.
Conclusion
The issue of healthcare professional burnout must be addressed for the sake of the patients, who deserve safe, high-quality care and for the health and well-being of the healthcare workers. Strategies to improve resilience must be developed and shared with all healthcare professionals.
Many healthcare employers offer physical and mental well-being programs at their workplace, however, few offer programs specifically address issues such as burnout. As more research becomes available, institutions should use this data to expand the support and outreach programs available to employees. Managers should ensure that their healthcare workers are aware of the resources that are available to them. In addition, those who are involved in the training of pharmacy students, residents, or fellows should be aware of the potentially deleterious effects of stress on performance and well-being and should refer them to appropriate resources at the college/institution to assist them.
Pharmacists and technicians who are ASHP members may be interested in joining the Clinician Well Being and Resilience community on ASHP connect to join the conversation on this issue and potentially share tips, advice, resources, and support. Finally, academicians and clinicians involved in research can assist by conducting more research in this area so that we can understand the issue more thoroughly and devise evidence-based solutions to help curb this epidemic.
The issue of healthcare professional burnout is an important one that deserves attention and action. Improving visibility of this issue at the organizational, state, and local levels is the first step in addressing this problem.
References:
1) Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012; 172:1377-85.
2) Shanafelt TD, Hasan O, Dyrbye L, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clinic Proc. 2015; 90:1600-13.
3) Jones JW, Barge BN, Steffy BD, et al. Stress and medical malpractice: Organizational risk assessment and intervention. J Appl Psychol. 1988;73:727-35.
4) Cimiotti JP, Aiken LH, Sloane DM, et al. Nurse staffing, burnout, and health care-associated infections. [Erratum appears in Am J Infect Control. 2012 Sep;40(7):680]. Am J Infect Control. 2012;40:486.
5) Welp A, Meier LL, Manser T. Emotional exhaustion and workload predict clinician-rated and objective patient safety. Front Psychol. 2015;5:1-13.
6) Halbesleben JRB, Rathert C. Linking physician burnout and patient ouccomes: Exploring the dyadic relationship between physicians and patients. Health Care Manage Rev. 2008;33:29-39.
7) McHugh MD, Kutney-Lee A, Cimiotti JP, et al. Nurses’ widespread job dissatisfaction, burnout, and frustration with health benefits signal problems for patient care. Health Aff. 2011;30:202-10.
8) Leiter MP, Harvie P, Frizell C. The correspondence of patient satisfaction and nurse burnout. Soc Sci Med. 1998;47:1611-7.
9) Vahey DC, Aiken LH, Sloane DM, et al. Nurse burnout and patient satisfaction. Med Care. 2004;42:1157-66.
10) DiMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: Results from the medical outcomes study. Health Pyshcol. 1993;12:93-102.
11) Waldman VS, Cruz Lopez Diez J, Arazi CH, et al. Burnout, perceived stress, and depression among cardiology residents in Argentina. Acad Psychiatr. 2009;33:296-301.
12) Gaither CA, Nadkarni A. Interpersonal interactions, job demands and work-related outcomes in pharmacy. Int J Pharm Pract. 2012, 20, pp. 80-89.
13) Le HM, Young SD. Evaluation of stress experienced by pharmacy residents. Am J Health-Syst Pharm. 2017; 74:599-604.
14) National Academy of Sciences. National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience. Available at: https://nam.edu/initiatives/clinician-resilience-and-well-being/. Accessed 2018 Feb 22.
15) Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014; 12: 573-576.
Professional Affairs - Call for Entries
Best Practice Award!

Past winners include:
2017
Sandra M. Salverson, Pharm.D., BCPS and Jerry Storm, RPh
Implementation of Integrated Telepharmacy Services Achieve a Health-System Standard of Pharmacy Care
2016
Maya Beganovic, PharmD and Sarah M. Wieczorkiewicz, PharmD, BCPS
"MALDI-TOF alone versus MALDI-TOF combined with real-time antimicrobial stewardship interventions on time to optimal therapy in patients with positive blood cultures"
2015
Kuntal Patel, Pharm.D., Pavel Prusakov, and Heather Vaule
"Osteopenia of Prematurity (aka Better Bones for Babies)"
2014
Arti Phatak, Pharm.D.; Brooke Ward, Pharm.D., BCPS; Rachael Prusi, Pharm.D.; Elizabeth Vetter, Pharm.D.; Michael Postelnick, BS Pharm, BCPS (AQ Infectious Diseases); and Noelle Chapman, Pharm.D., BCPS
"Impact of Pharmacist Involvement in the Transitional Care of High-Risk Patients through Medication Reconciliation, Medication Education, and Post-Discharge Callbacks"
View 2008 - 2013 Winners at ichpnet.org
Online entry form: http://www.ichpnet.org/professional_practice/best_practices/
Submission deadline: July 1, 2018
The objective of the Best Practice Award program is to encourage the development of innovative or creative pharmacy practice programs or innovative approaches to existing pharmacy practice challenges in health systems within the state of Illinois.
Applicants will be judged on their descriptions of programs and practices employed in their health system based on the following criteria:
- Innovativeness / originality
- Contribution to improving patient care
- Contribution to institution and pharmacy practice
- Scope of project
- Quality of submission
Eligibility
Applicants must be a member of ICHP for a minimum of 90 days prior to the submission deadline and practice in a health system setting. More than one program can be submitted by a health system for consideration.
Instructions for preparing manuscript
Each entry for the Best Practice Award must include a manuscript prepared as a Word document, double-spaced using Times New Roman 12-pitch type. A header with the paper title and page number should appear on each page. The manuscript should not exceed 2000 words in length (not counting references), plus no more than a total of 6 supplemental graphics (tables, graphs, pictures, etc.) that are relevant to the program. Each picture, graph, figure, and table should be mentioned in the text and prepared as a separate document clearly labeled.
The manuscript should be organized as a descriptive report using the following headings:
- Introduction, Purpose, and Goals of the program
- Description of the program
- Experience with and outcomes of the program
- Discussion of innovative aspects of programs and achievement of goals
- Conclusion
Format
Submissions will only be accepted via online submission form. The manuscript will be forwarded to a pre-defined set of reviewers. Please do not include the names of the authors or affiliations in the manuscript to preserve anonymity.
All applicants will be notified of their status within three weeks of the submission deadline. Should your program be chosen as the winner:
- The program will be featured at the ICHP Annual Meeting. You will need to prepare a poster to present your program and/or give a verbal presentation. Guidelines will be sent to the winner.
- You will be asked to electronically submit your manuscript to the ICHP KeePosted for publishing. This program will be accredited for CPE and will require that you complete material for ACPE accreditation.
- You will receive a complimentary registration to the ICHP Annual Meeting, recognition at the meeting and a monetary award of $1,000 distributed to your institution.
Non-winning submissions may also be considered for publication in the ICHP KeePosted, but your permission will be obtained beforehand.
If you have any questions related to the program please contact Trish Wegner at trishw@ichpnet.org.
Thank you to PharMEDium, Division of AmerisourceBergen for providing a grant for this year's Best Practice Award!
The 2016 & 2017 Best Practice Winners Available for CPE!
MALDI-TOF alone versus MALDI-TOF combined with real-time antimicrobial stewardship interventions on time to optimal therapy in patients with positive blood cultures
Authors: Maya Beganovic, PharmD and Sarah M. Wieczorkiewicz, PharmD, BCPS
Advocate Lutheran General Hospital, Park Ridge, Illinois
Implementation of Integrated Telepharmacy Services Achieve a Health-System Standard of Pharmacy Care
Authors: Sandra M. Salverson, PharmD, BCPS and Jerry Storm, RPh
OSF Healthcare System, Peoria, Illinois
Click here to learn how ICHP members can obtain free CPE credit.
Features
Call for Nominations
Lead, Follow or Get out of the Way! How Would You Like to Run for an ICHP Office?
Feature Article
by Scott A. Meyers, Executive Vice President
Have you ever observed a group with a purpose? Every effective group has a leader or two, a bunch of people ready to do whatever is needed and a few people who stand around and watch what happens. Every year, ICHP elects new members to its Board of Directors – the Leaders. Those are the folks we will begin searching for over the next couple of months.
Don’t get me wrong, we need a lot of those group members who are ready to do whatever the group has as a goal and in ICHP’s case that goal or mission is “Advancing Excellence in Pharmacy!” We recruit these folks all year long! We’ll call them Followers. Oh, and the Watchers, most often eventually become the Followers, ready to work on the goals and well, you can see where I’m headed.
So, every year the ICHP Committee on Nominations sifts through the Followers searching for new Leaders to step up to carry on the business of the Council and who are already dedicated to “Advancing Excellence in Pharmacy!”
This year is no exception. With Charlene Hope completing her term as Immediate Past President, ICHP will need someone to enter the top leader rotation as President-elect, which leads to President and eventually Immediate Past President over three years. In addition, Board Members Kathryn Schultz, Chris Crank, Mary Lee, Karin Terry, Elise Wozniak, and Amy Boblitt have a year left on their current terms and can run for another term for their respective offices if they choose. And even if they do decide to run again, the Committee on Nominations will be seeking a second candidate to make the ballot complete. Lynn Fromm will complete her second term as Southern Region Director next year so we will need both candidates for that position. And every year we need two energetic New Practitioners to step up and run for the New Practitioners Network Chair-elect. By the way, since 2003 when we formed the New Practitioners Network, several of the NPN Chairs have gone on to serve in a variety of offices within ICHP and other state societies!
Below is the complete list of the offices open for election in the fall of 2018. All the elected candidates will take office at the 2019 Annual Meeting in September with the exception of the President-elect, who assumes office immediately. So each new leader will have almost a year to train for their new jobs and be coached by our current Board Members. You don’t have to start leading right away!
President-elect
Treasurer-elect
Director-elect of Division of Government Affairs
Director-elect of the Division of Organizational Affairs
Director-elect of Division of Professional Affairs
Northern Region Director-elect
Central Region Director-elect
Southern Region Director-elect
NPN Chair-elect
If you are interested in running for an office or you would like to know more about an office before committing to run, you may contact Committee on Nominations Chair, Charlene Hope at chope@macneal.com or Scott Meyers at scottm@ichpnet.org. We hope you are ready to become an ICHP and Pharmacy Leader!
Meet Xing Jin
Feature Article
by Xing Jin, Doctor of Pharmacy Candidate 2018, UIC - Rockford
My name is Xing Jin and I am a fourth-year pharmacy student from the UIC Rockford campus. I was born in China and moved to the United Stated with my parents when I was thirteen. I lived in North Dakota for five years and after graduating high school I went to the University of Wisconsin – Madison. I graduated in 2013 with a double major in Biology and Communication Arts. I was accepted to the Doctor of Pharmacy program at University of Illinois – Chicago and I have lived in Rockford since then.
You Want Me to Talk to a Legislator???
Feature Article
by Kathryn Schultz, PharmD, BCPS, BCOP, ICHP Treasurer and member of Division of Government Affairs; Michaela Murphy, PharmD Candidate 2020, Rush University Medical center, Pharmacy Technician Intern; Katie Dalton, PharmD Candidate 2019, Rush University Medical Center, Pharmacy Technician Intern
On Wednesday, March 7, 2018, about 400 pharmacists, pharmacy students, and pharmacy technicians visited Springfield, IL for “Illinois Pharmacy Legislative Day: Under the Dome” during the 100th Illinois General Assembly to speak with legislators regarding important pharmacy issues. While visiting the Capitol, we not only met with our legislators but we also listened to the House of Representatives while they were in session. It was interesting to observe the operations of our legislative process first hand and very rewarding to be able to personally educate our legislators on the impact these bills would have on our profession. The four bills of focus for 2018 are summarized below along with recommendations for your legislator on how to vote on these issues.
1) HB5442: Clogging the PMP with Unnecessary Information. This bill amends the Controlled Substance Act to require hospitals to report all controlled substances ordered and administered to patients to the Prescription Monitoring Program (PMP).
a. However, this reporting is likely unnecessary and takes away from the purpose of the PMP, which is to monitor for patients who are “provider shopping” and potentially abusing controlled substances. Patients are not able to “provider shop” within the hospital and are closely monitored by multiple disciplines (including pharmacy) for appropriate usage.
b. Adding all these inpatient orders would clog up the PMP! Recommend to your legislator to VOTE NO!
2) HB3285/SB1844: Extending Pharmacy Rights by Instituting Audit Protections. This bill amends the Illinois Insurance Code to provide fair guidelines on pharmacy audits by Pharmacy Benefit Managers (PBMs).
a. In the state of llinois, one has to undergo the rigorous process of registering as a PBM and pay $49 to start auditing pharmacies and “recouping” payment for insurance companies.
b. The purpose of a PBM should be to audit for fraud, waste, and abuse of insurance payments. However, PBMs in their current state, are completely unregulated and can charge-back payments for computer or clerical errors that had no negative impact on the patient.
c. Recommend to your legislator to VOTE YES and extend pharmacy rights by instituting audit protections.
3) HB3479/SB1888: Preserving Pharmacies in Our Communities and for Our Patients. This bill amends the Illinois Public Aid code to provide fair reimbursement within the Managed Care Organization (MCO) contracts with the state of Illinois for Medicaid.
a. Previously, Medicaid reimbursed based on a Fee-For-Services methodology. In 2016, a pilot program was started that attempts to improve health outcomes while controlling health care costs. This new program utilized contracts with MCOs to provide insurance coverage to the ~25% of the residents of Illinois who are on Medicaid.
b. Analysis of the pilot program has shown a significant reduction in the reimbursement of prescriptions dispensed and pharmacist-provided patient care services are well below acquisition costs (how much it costs the pharmacy to purchase the drug).
c. This bill provides guidance for fair reimbursement so that it is not below the acquisition cost and a fair and reasonable professional fee to be established for pharmacist-provided patient care services.
d. It also requires improved documentation of dollars handled by the MCOs (who paid who and how much) as there is currently a report that $7.1 billion dollars is unaccounted for in 2016 alone.
e. Please VOTE YES to mandate that MCOs contract with the Illinois Department of Healthcare and Family Services (HFS) to establish, maintain, and provide a fair and reasonable reimbursement rate to pharmacy providers for prescriptions dispensed and pharmacist-provided patient care services.
4) HB5747: Improving Women’s Access to Hormonal Contraception. This bill amends the Pharmacy Practice Act to allow pharmacists to provide patients with increased access to hormonal contraceptives.
a. Pharmacists with specialized training will be able to provide hormonal contraceptive patches, vaginal rings, and self-administered oral hormonal contraceptives after a patient’s medical history is screened. Access to contraceptives is crucial in reducing unintended pregnancies.
b. The contraceptives would continue to be covered by insurance.
c. Currently Oregon, California, Colorado, New Mexico, Maryland, and Hawaii all have programs that allow pharmacists to prescribe contraception. It is estimated that having pharmacists in this role could decrease the number of unintended pregnancies by 7-25%.
d. Please VOTE YES to expand women’s access to hormonal contraceptives!
So Now What?
We cannot solely rely on one day in Springfield to advocate for our profession! If you don’t know who your legislator is, look them up here:
http://www.elections.illinois.gov/DistrictLocator/DistrictOfficialSearchByAddress.aspx
Become an advocate for our profession – legislators want to hear from you! Contact them and educate them on the impact these bills will have on our profession and our patients. Most of them do not understand what a hospital pharmacist does, so invite them to see what hospital pharmacy is all about! In November 2017, we invited Representative Sonya Harper to come to Rush University Medical Center for a short visit. This was a great experience for everyone!
How Can You Set up a Visit?
First, get approval from your director of pharmacy and determine who else at the institution needs to approve the visit before arranging it. For our institution, we needed the approval of the Vice-President of the health system. We also reached out to public affairs to confirm whether they needed to be notified and we were asked to have a visitor HIPAA confidentiality form signed by the representative. We emailed the representative and worked with her local assistant to set up the time and location and relay the need for the HIPAA form to be signed.
Be patient with setting up these appointments! The representative may need to cancel and reschedule their visit, since their schedules are ever changing. We had to reschedule 3 times, but we were understanding of her need to reschedule and it finally worked out! Keep the visit SHORT! There are so many people wanting the ear of these legislators. We kept our visit to 1 hour. During that visit we showed her what interdisciplinary patient care rounds are about and how the pharmacist can have a role in these. Be sure to prep your physicians that they may encounter the legislator. We were fortunate to have a very strong advocate for pharmacists with the physician leading rounds so he even had a direct conversation with Representative Harper about the value he sees in having a pharmacist on rounds! We then took her on a tour to a pharmacy satellite and to the central pharmacy. We described how we incorporate technology into our work, our work with pharmacy technicians, and how important the technician’s role is for hospital pharmacy. Finally, we had her observe our daily huddle—essentially a 10-15 minute sign-out from day shift to evening shift of any issues and updates on drug shortages.
 What’s next? Invite more legislators to see what hospital pharmacy is all about! Representative Harper commented on how her eyes had been opened to a whole new aspect of pharmacy. We were then able to meet with her again in Springfield for Legislative Day. She recognized us and we were able to have a great conversation with her about the current bills.
What’s next? Invite more legislators to see what hospital pharmacy is all about! Representative Harper commented on how her eyes had been opened to a whole new aspect of pharmacy. We were then able to meet with her again in Springfield for Legislative Day. She recognized us and we were able to have a great conversation with her about the current bills.
We encourage you to reach out to your legislators and invite them to visit your hospital so that we can continue to educate them on the practice of hospital pharmacy. This better understanding of what pharmacists and pharmacy technicians do will be of value in the near future when there are bills that impact our practice and especially as we work to propose changes to the Pharmacy Practice Act before it sunsets.
If you are looking to become even more involved, you can also consider joining the ICHP Division of Government Affairs to be a proponent of the change for our profession!
College Connection
Midwestern University Chicago College of Pharmacy
Reflections of a Student Pharmacist
College Connection
by Ziad Dabbagh, PS-2, ICHP Historian
Will Assibey, Jr. is a PharmD candidate who is studying at Midwestern University Chicago College of Pharmacy. Through his reflections on his past experiences, I learned about his involvement on campus and how his activities relate to where he wants to take is pharmacy career. Beginning pharmacy school in the Fall of 2015, Will has had a variety of experiences which he was willing to reflect on during our interview in hopes that they will aid other students who may be interested in becoming more involved with organizations during pharmacy school.
Our interview began with an explanation of past experiences as they related to his first year as a pharmacy student. “I wasn’t very sure where I wanted to go in my career, like many students, so I decided to join organizations,” explained Will. During his first year, he was a member of many organizations. Will explained that it was easy to become overwhelmed with the amount of information and the multitude of directions that each organization could take a person. Through various campus activities which encouraged involvement and self-discovery, Will was able to whittle down his interests to four organizations in which to immerse himself. Will chose to become involved in Phi Delta Chi (PDC; to foster camaraderie with other students), the American Pharmacists Association – Academy of Student Pharmacists (APhA-ASP; due to his interest in pharmacy as a profession), the American Association of Pharmaceutical Scientists (AAPS; to gain some insight into the pharmaceutical industry), and the Illinois Council of Health System Pharmacists (ICHP; to gain a better understanding of pharmacy and how it is practiced in Illinois). Will spent the “majority of [his] first year getting to know others and picking their minds regarding school.”
Progressing to a second-year pharmacy student and with more refined interests, Will continued his involvement with all of the same organizations, except now taking on more responsibility as an elected board member for APhA-ASP and PDC. One particular project Will enjoyed was being a volunteer for the Medication Therapy Management / Collaborative Healthcare Advocacy Team (MTM/CHAT) in Bolingbrook, Illinois. MTM/CHAT is a collaborative effort at a healthcare center in Bolingbrook which enables students to give back to the community by practicing medication therapy management services and gaining experience by counseling patients about diabetes. Asked to comment on his second-year, Will had to say that aside from studying his “roles have taken a better portion of [his] time which has limited [him] from being involved with other organizations.” Recalling the time spent being involved with APhA-ASP and ICHP during his second-year, Will enjoyed when these organizations brought in professionals to share their insight on different areas of pharmacy; he also shared with me that it is his goal to one day speak about his experiences in pharmacy.
Although this is now Will’s third year in pharmacy school, he recollects his memories about things he has experienced thus far for the purpose of sharing his perspective. This year, Will has “learned how to taken charge of projects while aiding in the improvement” of organizations in which he is involved. Organizations such as PDC, of which he is currently the president, along with APhA-ASP where he serves as the Membership Chair. Recently, he was involved in the promotion of an event for APhA-ASP which brought students and residency directors together to discuss residencies and answer any questions students may have regarding post-graduate education.
While only time will tell what happens in Will’s future, he hopes to stay involved and muses over what a fourth year pharmacy student’s life may be like and the things that he may learn. Hoping that “rotations will give more insight on what the real world is like,” he realizes that so far, he seems to have a strong interest in clinical pharmacy and plans on pursuing this route as a long-term career path. Until then, it is Will’s goal to apply for a residency in this area and stay up-to-date with the profession.
Roosevelt University College of Pharmacy
New Updates and Upcoming E-board Elections
College Connection
by Anna Majerczak, PS3, SSHP Webmaster and Membership Chair & Hayden Hagemann, PS-3, Treasurer
Our SSHP chapter at Roosevelt University has been quite busy these past few weeks and has a lot of events planned for our coming term! We have recently added a PAI subcommittee to our organization and welcomed two new members to our Executive Board. We have also worked in conjunction with other student organizations within our College of Pharmacy to promote student involvement, professional development, and raise money for charity.
We hosted our spring journal club on the article, “Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis”. There was a great turn-out with 21 attendees, including three clinical faculty members, and lively discussions that had the whole room involved. To close out our spring term, we have a CV workshop set up that will include several of the clinical faculty at Roosevelt. Members will have the opportunity to send their CV in to our Executive Board, and we will then evenly distribute them out for faculty review. This event is very timely as our P3 students are moving closer to APPE rotations and career/residency applications.
Dr. Matt Dandino, PharmD, BCPS, from Mount Sinai Hospital in Chicago, IL is coming to speak to our organization about a process he worked on to streamline a transitions of care service line. This is a great opportunity for students to learn more about a topic in which pharmacists can play a huge role. We are also gearing up for our annual residency round table event by reaching out to Northwestern Memorial Hospital, Rush University Medical Center, Advocate Christ Hospital, and Mount Sinai Hospital to invite current pharmacy residents to RUCOP to answer questions regarding their path to residency. Our last residency round table event was extremely successful and we are hoping this one will be the same.
The SSHP worked concurrently with our student government to sponsor a hot chocolate bar during a finals week De-Stress Fest. De-Stress Fest was created to help fellow students relax and take a break during the most stressful time of the term. We are currently working with Kappa Psi to fundraise for St. Baldrick’s Foundation to raise money and promote awareness for childhood cancer. We made a “pot of gold” basket to raffle off at a trivia night hosted by our student government. Finally, we are also giving back to the community by volunteering at Feed My Starving Children. We are looking forward to volunteering and helping those in need with other classmates and our faculty.
Looking to the next academic year, we are in the process of holding Executive Board elections. We are running elections earlier than in the past to allow the new Executive Board more time to shadow and train under our wing. We hope by electing our new Executive Board early it will allow them to be better prepared to take over next year. We are extremely excited to get our new Executive Board training and hear their ideas for next years’ events.
Rosalind Franklin University
Giving Back at RFU
College Connection
by Joseph DeSantis College of Pharmacy Class of 2019, ICHP Treasurer
At Rosalind Franklin University, our ICHP chapter puts great emphasis on patient advocacy and community outreach. During the Winter quarter, we wanted to extend our patient outreach to a population we thought could use more attention. We contacted a local elementary school in Waukegan, IL, and participated in the “Kicking Asthma” event sponsored by the American Lung Association. The goal of this event was to educate children on how to manage asthma and prevent asthma exacerbations. It is important for children of this age to learn about asthma, not only because it might affect them personally, but they might also have friends or family who are affected as well. Most students that attended were referred by the school nurses that are diagnosed with asthma but students without an asthma diagnosis were in attendance.
The event was divided into 4 different sessions, each with their own learning objectives. In the first session, we introduced and reviewed the basics of asthma and provided general information about how asthma exacerbations affect the lungs. In addition, we emphasized recognizing common signs and symptoms of an asthma attack. Lastly, we asked participants to identify personal goals that can help them to maintain control of their asthma.
The second session focused on preventing asthma exacerbations and identifying asthma triggers and warning signs. This session allowed participants to identify their own specific triggers and signs that an asthma attack is imminent to help them either avoid or prevent an asthma exacerbation. During this session we emphasized the importance of regular doctor visits for follow-up and medication review.
The third session focused on medications to control asthma. We discussed how each medication works for treating asthma and preventing exacerbations. Proper technique for specific inhalers was demonstrated for each inhaler and we discussed proper storage requirements. We stressed the importance of medication adherence, as this can help prevent asthma symptoms and exacerbations.
The fourth and final session focused on tying everything together. We taught the participants what to do in emergency situations and helped them find solutions to problems if something in their asthma plan did not go exactly as expected.
This event was a great way for us to build a relationship with our community while being able to help future generations. The children who we interacted with were all very excited and happy to attend each session. A lot of healthcare professionals do not have the opportunity to interact with the pediatric population. I would recommend this to everyone because children are not only a joy to be around but they are our future as well.
More
Officers and Board of Directors
Executive Officers
 Travis Hunerdosse
Travis Hunerdosse
President
(312) 926-6124
thunerdo@nm.org
 Charlene Hope
Charlene Hope
Immediate Past President
(708) 783-5933
chope@macneal.com
 Noelle Chapman
Noelle Chapman
President-Elect
 Kathryn Schultz
Kathryn Schultz
Treasurer
(312) 926-6961
kathryn_schultz@rush.edu
Regional Directors
 Amy Boblitt
Amy Boblitt
Regional Director Central
(217) 788-3015
boblitt.amy@mhsil.com
 Elise Wozniak
Elise Wozniak
Regional Director Northern
elise.m.wozniak@gmail.com
 Lynn Fromm
Lynn Fromm
Regional Director
Southern
(618) 391-5539
fromml@andersonhospital.org
Division Directors
 Mary Lee
Mary Lee
Organizational Affairs Director
(630) 515-7311
mleexx@midwestern.edu
 Karin Terry
Karin Terry
Professional Affairs Director
(309) 655-3390
Karin.l.terry@osfhealthcare.org
 Lara Ellinger
Lara Ellinger
Educational Affairs Director
(312) 926-3571
laelling@nm.org
 Carrie Vogler
Carrie Vogler
Marketing Affairs Director
(217) 545-5394
cvogler@siue.edu
Technician Representative
Network and Committee Chairs - non-voting
 Bernice Man
Bernice Man
Chairman, New Practitioners Network
(773) 702-9641
bernice.man.pharmd@gmail.com
 Abby Kahaleh
Abby Kahaleh
Ambulatory Care Network Chair
akahaleh@roosevelt.edu
 David Tjhio
David Tjhio
Chairman, Committee on Technology
(816) 885-4649
david.tjhio@cerner.com
 Jennifer Phillips
Jennifer Phillips
Editor & Chairman KeePosted
Committee Chair,
Nominations Committee
(630) 515-7167
jphillips@midwestern.edu
 Milena McLaughlin
Milena McLaughlin
Assistant Editor, KeePosted News Journal
(630) 515-7293
mmclau@midwestern.edu
Student Chapter Presidents
Ashley Shinnick - Chicago State University College of Pharmacy
Maggie Lau - Midwestern University Chicago College of Pharmacy
Kimberly Zaleski - Roosevelt University College of Pharmacy
Aprille Banchoencharoensuk - Rosalind Franklin University
College of Pharmacy
aprille.banchoencharoensuk@my.rfums.org
Kaylee Poole - Southern Illinois University Edwardsville School of Pharmacy
David Silva - University of Illinois at Chicago College of Pharmacy
HyeRim Whang Kong - University of Illinois at Chicago - Rockford Campus College of Pharmacy
ICHP Affiliates
Northern Illinois Society (NISHP)
Erika Hellenbart
President
Denise Kolanczyk
President-elect
Antoine Jenkins
Immediate Past President
(773) 821-2592
David Martin
Treasurer
Milena McLaughin
Secretary
(630) 515-7293
Vera Kalin
Technician Representative
West Central Society of Health-System Pharmacists (WSHP
Ed Rainville
President
ed.c.rainville@osfhealthcare.org
Metro East Society (MESHP)
Jared Sheley
President
Sangamiss Society of Heath-System Pharmacists
Julie Downen
President
217) 788-3953
Billee Samples
President-elect
Katelyn Conklen
Immediate Past President
Vacant Roles at Affiliates; President, Rock Valley Society; Southern IL Society; Sugar Creek Society
Follow us! Find us here:
Upcoming Events
Visit the ICHP Calendar for the most up-to-date events! 
Save the Date! Watch for more information in upcoming news briefs.
NISHP Double Feature
WildFire Chicago
159 W. Erie St., Chicago, IL
New Treatment Options for Distributive Shock
Scott Benken, PharmD, BCPS-AQ Cardiology
Clinical Assistant Professor, University of Illinois at Chicago College of Pharmacy
Clinical Pharmacist, Medical and Cardiothoracic Surgery Intensive Care
University of Illinois Hospital and Health Sciences System
(Presentation #1 is Sponsored by La Jolla Pharmaceutical Company)
Presentation #2:
Women's and Men's Health Updates: Focus on Hormonal Therapy
Brooke Griffin, PharmD, BCACP
Professor of Pharmacy Practice and Vice Chair for Clinical Services
Midwestern University Chicago College of Pharmacy
and
Drew Halbur, PharmD
Comprehensive Pharmacy Services
Clinical Specialist, Ambulatory Care
(Accredited for pharmacists and pharmacy technicians | 1.0 contact hour (0.1 CEU)
(Presentation #2 is Sponsored by ICHP)
Program seating is limited with only 10 slots available for students.
ICHP Pharmacy Action Fund (PAC) Contributors
ICHP Pharmacy Action Fund Contributors:
Welcome New Members!

| New Member | Recruiter |
| Eric Abasolo | |
| Jenine Abuzir | |
| Alyshia Accardi | |
| Audra Andersen | |
| Lynda Azubuike | |
| Shawna Baumgartner | |
| Isaiah Blackburn | |
| Erin Budris | |
| Maulik Dave | |
| Palak Desai | |
| Maria Doan | |
| Kayla DuBois | |
| Souraya EL-Sayed-Abdallah | Daniel Castro |
| Rachel Geschwill | |
| Daniel Gucwa | Ani Bekelain |
| Famia Habeeb | |
| Rebecca Ham | Bridget Dolan |
| Paige Harmon | |
| Craig Hernandez | |
| Giacang Hoang | |
| Jaime Holden | |
| Wing Yee Ip | |
| Steven Jannick | |
| Jeremy Jones | |
| Derek Joseph | Florence Patino |
| Karen Juco | |
| Natalie Julius | |
| Paul Kalhon | |
| Natalia Kapusciak | |
| Agnieszka Karpa | |
| Noura Keshta | |
| David Kidder | |
| Amanda Kojda | |
| Joanna Kozien | |
| Patrick Kuchta | |
| Shweta Kurma | |
| Jismon Kuruppath | |
| Anthony Labriola | |
| Kimberly Lenaway | |
| Sara Lindquist | Ashley Shinnick |
| Paden Long | |
| Adela Lupas | |
| Neil Miran | |
| Maryam Naveed | |
| Nhung Nguyen | |
| Phuong Nguyen | |
| Katherine Nowak | |
| Ashor Oshana | |
| Rita Piro | |
| Lisa Potter | |
| Jessica Rinehart | |
| Erin Rogovic | Lisa Dubien |
| Kenneth Rowe | |
| Camisha Ruffins | |
| Monica Salabun | |
| Joab Salvador | |
| Nimita Shah | |
| Daryl Truong | |
| Elyse Vesely | |
| Amanda Walsh | Denise Lamm |
| Meghan Walsh | |
| Krystian Wojdyla | |
| Nicholas Wright-Hart | |
| Paul Wyzkiewicz | |
| Bahareh Zakeri | |
| Jenny Zhao |







 Jennifer Arnoldi
Jennifer Arnoldi Scott Meyers
Scott Meyers Christopher Crank
Christopher Crank Clara Gary
Clara Gary