Official Newsjournal of the Illinois Council of Health-System Pharmacists
Educational Affairs
Baricitinib EUA Shakes Up COVID-19 Therapy: What You Need To Know
by Dan Majerczyk, PharmD, BCPS, BC-ADM, CACP; Assistant Professor of Clinical Sciences - Roosevelt University, College of Science, Health and Pharmacy - Schaumburg, IL; Clinical Pharmacy Specialist - Loyola Medicine -MacNeal Family Medicine Residency Program - Berwyn, IL; Janki Vyas, PharmD Candidate 2022 Student - Southern Illinois University Edwardsville School of Pharmacy - Edwardsville, IL; Part-time Pharmacy Intern - South City Hospital/CVS Pharmacy - St. Louis, MO; Ashley Stefanski, PharmD Clinical Instructor, Academic Fellow Roosevelt University, College of Science, Health and Pharmacy - Schaumburg, IL
.jpg)

- Centers for Disease Control and Prevention. CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/ (accessed 2021 Feb 23).
- Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
- Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656-665.
- Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020.
- Baricitinib [monograph]. In: Lexicomp Online [online database]. Hudson, OH: Lexi-Comp (assessed 2020 Jan 6).
- Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462:1-13.
- Bousoik E, Montazeri Aliabadi H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front Oncol. 2018;8:287.
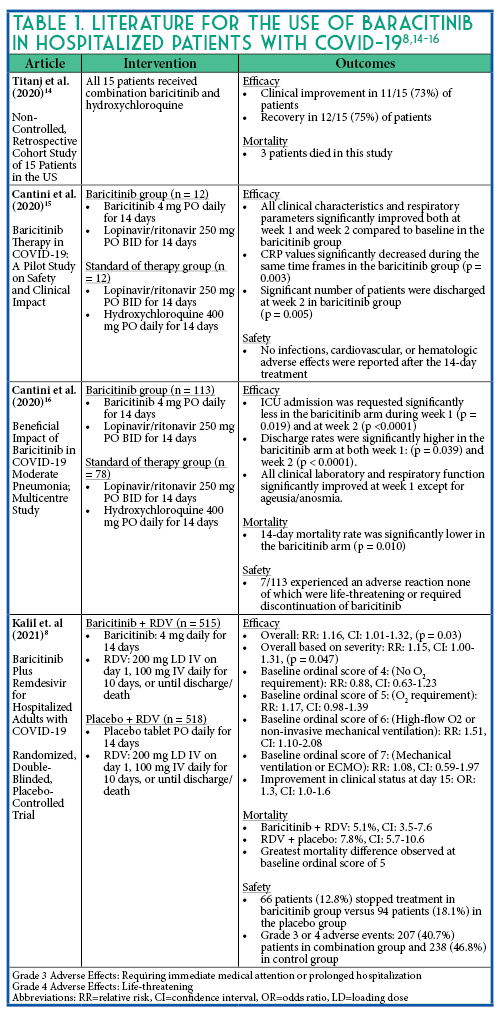
- Kalil AC, Patterson TF, Mehta AK et al., Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. Randomized, double-blind trial evaluating baricitinib plus remdesivir in hospitalized patients with Covid-19. New Engl J Med;2021.384(9):795-807.
- Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393.
- Jorgensen S, Tse C, Burry L, Dresser L. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy. 2020;40:843-856.
- Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400-402.
- Food and Drug Administration. Commissioner of the U.S. Food and Drug Administration. http://www.fda.gov/ (accessed 2021 Feb 23).
- Remdesivir [monograph]. In: Lexicomp Online [online database]. Hudson, OH: Lexi-Comp (assessed 2020 Jan 6).
- Titanji BK, Farley MM, Mehta A, et al. Use of Baricitinib in Patients With Moderate to Severe Coronavirus Disease 2019. Clin Infect Dis. 2020:ciaa879.
- Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. 2020;81:318-356.
- Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81:647-679.
Contents
Columns
PEARLS: Public Education & Awareness Outreach Publication Subcommittee
Features
PTCB Credentials & Certificates
Spring Meeting 2021: Poster & Platform Winners
College Connection
Midwestern University College of Pharmacy, Downers Grove
Southern Illinois University Edwardsville School of Pharmacy
University of Illinois Chicago College of Pharmacy
More
ICHP Pharmacy Action Fund Contributors
ICHP Board of Directors 2021-2022
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.

.jpg)