Official Newsjournal of the Illinois Council of Health-System Pharmacists
Opioid Task Force - CPE Opportunity
How to Assess Health-System Implementation of the CDC Recommendations for Prescribing Opioids
by Mark E. Greg, PharmD, RPh Director, Ambulatory Pharmacy Management, Northwestern Medicine Physician Network Oak Brook, IL
- Obtain leadership support as a critical first step
It is likely appropriate opioid-related initiatives are currently taking place within your organization. If not, you may be the catalyst for that initiative. Leadership support may be obtained from various departments including administration, pharmacy, nursing, medical director, quality, physician practices, emergency department, immediate care, and others.
Regardless of your role, a first step may be to ask your manager or other key contacts within your organization where appropriate opioid prescribing falls as an organizational priority. Perhaps there is an active committee examining pain management or opioid prescribing. If not, you may be the person to promote this as a Pharmacy & Therapeutics, Quality Improvement, Utilization Management, Patient Safety, Emergency Medicine, Immediate Care Center initiative, or Ambulatory Primary Care Initiative. Leadership reacts to metrics including quality, cost-avoidance, and financial performance.
- Identify a champion(s) to drive the change process
In general, an administrative champion or one or more physician champions are key individuals to engage. Granted, in many cases, pharmacists may be the drivers of quality improvement initiatives and are highly respected by physicians, yet physicians may be more likely to listen to and accept direction from fellow physicians. Securing physician champion support may be aided by informing them that they work will be shared among the team. That is the role of the multidisciplinary change or quality improvement team as discussed below.
- Form a change team (if appropriate) or at least engage key staff
Representatives may include providers (e.g., physicians, advanced practice nurses, physician assistants, pharmacists), nursing, quality, administration, electronic health record support, and IT/analytics. Your providers will be able to share what their needs are based upon the challenges they encounter in daily practice. Meetings involving the physician champion(s) may require early morning or late afternoon times to accommodate clinic hours/patient care activities. Ongoing collaboration between system inpatient and ambulatory opioid initiatives is highly recommended.
- Obtain needed resources and determine readiness
Key resources will include meeting time, a meeting location/virtual meeting, IT/analytics support, EHR support, and data. Key questions to ask your champions and key participants include, “Does the organization identify a problem with current pain management/opioid prescribing?” and “Is the organization willing to make changes in pain management practices?” Changing culture takes time.
- Assess current policies and practices
Does the organization have any? If not, create your own. What do you need? How detailed do you want them to be? What do your providers need to make them successful? What have your peers in other health systems developed? The internet includes postings by many health systems that may serve as policy templates.
- Complete the self-assessment questionnaire
An organizational self-assessment is included in Appendix C (pages 52-56) of the guidelines.
- Collect data on your patient population and opioid therapy
Decide what data you wish to collect. What opioids are being prescribed? What is the morphine milligram equivalent (MME) count? Are opioids in combination with benzodiazepines being prescribed? Is naloxone being prescribed for at-risk patients? Your EHR may be able to capture prescription claims data. Some organizations may also receive prescription claims data or reports from payer partners. Review of data may require manual calculation of morphine milliequivalents per day as well as screening for other concomitant CNS-depressant medications.
- Determine access to specialists and other resources
What resources are available? Mental health, pain specialist, substance use programs, and providers of Medication Assisted Therapy (MAT) are just a few important resources. If providers are uncertain how to connect patients requiring substance abuse treatments with available resources, a major “win” may be for the organization to prepare and provide printed lists of substance use providers or EHR links for referrals to MAT providers.
- Identify areas for improvement
What are your greatest needs? Reporting data will accomplish the following: 1) provide baseline measure performance; 2) identify outliers (under- and over-prescribers); and 3) identify those prescribers and patients who may benefit from opioid prescribing assistance. Use the data to help guide your team.
- Determine which guideline recommendations to implement
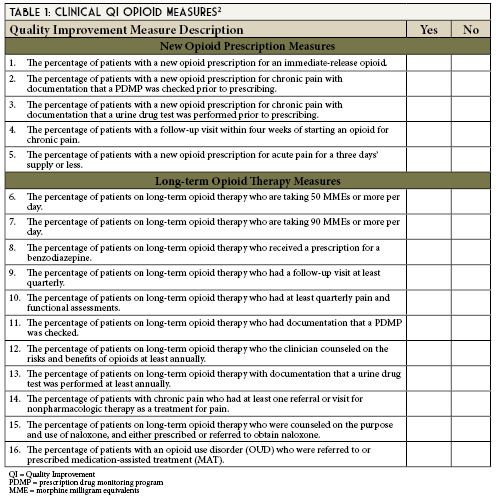
Appendix B (pages 33-50) of the guidelines entitled, Operational Clinical Quality Improvement (QI) Opioid Measures provides short and long-term opioid measure operational definitions. Operational definitions include the criteria used to build reports. These will assist your data and analytics architects when creating your reports.
- Prioritize what will be implemented
What will you be able to accomplish within your organization? What is the most pressing need? Be realistic given the resources available within your organization and expected time frame to see results.
- Set measurable goals
These goals will vary based upon your baseline data and what may be realistically accomplished within your organization. Administrative and physician leadership prefer metrics.
- Develop a plan for implementing selected guideline recommendations
- Implement the changes
- Monitor progress using QI measures and other data
- Review metrics for assessment of opioid prescribing practices
Case 1 Discussion
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1-49. doi: http://dx.doi.org/10.15585/mmwr.rr6501e1
- Centers for Disease Control and Prevention. Quality Improvement and Care Coordination: Implementing the CDC Guideline for Prescribing Opioids for Chronic Pain. 2018. National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention, Atlanta, GA. Available at: https://www.cdc.gov/drugoverdose/pdf/prescribing/CDC-DUIP-QualityImprovementAndCareCoordination-508.pdf. Accessed June 6, 2020.
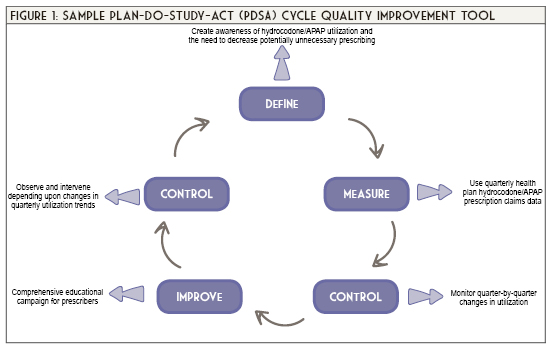
- Plan-Do-Study-Act (PDSA) Worksheet. Institute for Healthcare Improvement. Cambridge, MA. http://www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed June 6, 2020.
- The Define, Measure, Analyze, Improve, Control (DMAIC) Process. American Society for Quality. https://asq.org/quality-resources/dmaic Accessed June 6, 2020.

Contents
Columns
Public Education & Awareness Outreach Publication Subcommittee
Attention ASHP Pharmacist Members
Professional Affairs - CPE Opportunity!
Features
ICHP's Newest Affiliate Leaders
Opioid Task Force - CPE Opportunity
College Connection
Midwestern University Chicago College of Pharmacy
Rosalind Franklin University College of Pharmacy
Southern Illinois University Edwardsville School of Pharmacy
University of Illinois Chicago College of Pharmacy
More
Pharmacy Action Fund Contributors
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.
