Official Newsjournal of the Illinois Council of Health-System Pharmacists
Educational Affairs
Use of Direct Oral Anticoagulant Medications For Atrial Fibrillation: Current Recommendations in Valvular Heart Disease
by Brian Cryder, PharmD, BCACP, CACP Associate Professor and Clinical Pharmacist; Midwestern University Chicago College of Pharmacy and Advocate Medical Group; Austin Ballew, PharmD, BCPS Ambulatory Care Clinical Pharmacist; Advocate Medical Group (Sykes Center)
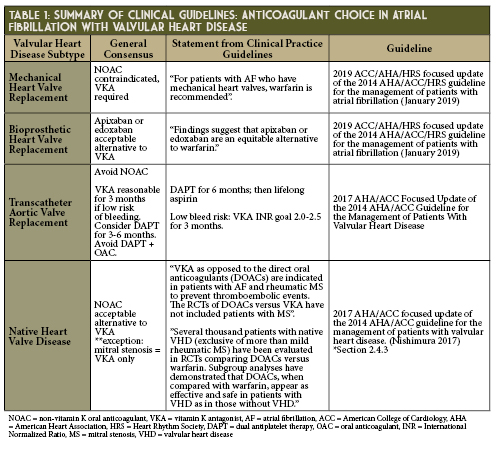
Warfarin has long been the sole oral anticoagulant used in patients with mechanical prosthetic valve (MPV) replacements, but in the past decade DOACs have been studied as an alternative. To date, no past DOAC studies have included patients with concomitant AF and MPV.
The phase 2 RE-ALIGN study sought to compare dabigatran (dosed to meet plasma trough levels > 50 ng/ml) to warfarin.3 RE-ALIGN primarily aimed to study the pharmacokinetics of dabigatran in this population, but the trial required early discontinuation due to excess in both thromboembolic and hemorrhagic complications when compared to warfarin. Researchers now attribute this therapeutic failure to localized thrombin generation triggering thrombosis in MPV-associated clotting rather than tissue factor as seen in non-valvular atrial fibrillation (NVAF) and venous thrombosis.3-5 Jaffer and colleagues discovered that MPV required dabigatran concentrations > 200 ng/ml to suppress thrombin generation to the same extent that warfarin does when maintained at INR levels between 2.0-3.5 thus RE-ALIGN’s target of 50 ng/ml was insufficient.4
Transcatheter Aortic Valve Replacement
Over the next decade, as the DOAC patents expire and become financially accessible to a larger pool of patients, it will be important to ensure efficacy and safety in the appropriate patient population. Few experts anticipate major changes in current guidelines for DOAC use in MPV, BPV and most VHD, but post-TAVR anticoagulation will be shaped largely by the arrival of new research over the next several years.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017; 135: e1159-e1195. DOI: 10.1161/CIR.0000000000000503
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation 2019; 140: e125-e151. DOI: 10.1161/CIR.0000000000000665
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013; 369: 1206-1214. DOI: 10.1056/NEJMoa1300615
- Jaffer IH, Stafford AR, Fredenburgh JC, et al. Dabigatran is less effective than warfarin at attenuating mechanical heart valve-induced thrombin generation. J Am Heart Assoc. 2015; 4: e002322. DOI: 10.1161/JAHA.115.002322
- Chan NC, Weitz JI, Eikelboom JW. Anticoagulation for mechanical heart valves: Will oral factor Xa inhibitors be effective? Arterioscler Thromb Vasc Biol 2017; 37: 743-745. DOI: 10.1161/ATVBAHA.117.309223
- Lester PA, Coleman DM, Diaz JA, et al. Apixaban versus warfarin for mechanical heart valve thromboprophylaxis in a swine aortic heterotopic valve model. Arterioscler Thromb Vasc Biol 2017; 37: 942-948. DOI: 10.1161/ATVBAHA.116.308649
- Roost E, Weber A, Alberio L, et al. Rivaroxaban in patients with mechanical heart valves: A pilot study. Thrombosis Research 2020; 186: 1-6. DOI: 10.1016/j.thromres.2019.12.005
- Duraes AR, Bitar YSL, Lima MLG, et al. Usefulness and safety of rivaroxaban in patients following isolated mitral valve replacement with a mechanical prosthesis. Am J Cardiol 2018; 122: 1047-1050. DOI: 10.1016/j.amjcard.2018.06.015
- Duraes AR, Bitar YSL, Filho JA, et al. Rivaroxaban versus warfarin in patients with mechanical heart valve: rationale and design of the RIWA study. Drugs in R&D 2018; 18: 303-308. DOI: 10.1007/s40268-018-0249-5
- Carvalho Silva DM, Braga A, de Jesus I, Neves J. Mechanical prosthetic heart valve thrombosis in a patient receiving rivaroxaban. Cardiology 2019; 143: 116-120. DOI: 10.1159/000501361
- Kumar V, Kelly S, Raizada A, et al. Mechanical valve thrombosis on rivaroxaban: Are novel anticoagulants really an option? Methodist Debakey Cardovasc J 2017; 13 (2): 73-75. DOI: 10.14797/mdcj-13-2-73
- Jaffer IH, Fredenburgh JC, Stafford A, Whitlock RP, Weitz JI. Rivaroxaban and dabigatran for suppression of mechanical heart valve-induced thrombin generation. Ann Thorac Surg 2020; epub ahead of print. DOI: 10.1016/j.athoracsur.2019.10.091
- Guimaraes PO, Pokorney SD, Lopes RD, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: Insights from the ARISTOTLE trial. Clinical Cardiology 2019; 42: 568-571. DOI: 10.1002/clc.23178
- DeCaterina R, Renda G, Carnicelli AP, et al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol 2017; 69 (11): 1372-1382. DOI: 10.1016/j.jacc.2016.12.031
- Malik AH, Yandrapalli S, Aronow WS, Panza JA, Cooper HA. Oral anticoagulants in atrial fibrillation with valvular heart disease and bioprosthetic heart valves. Heart 2019; 105: 1432-1436. DOI: 10.1136/heartjnl-2019-314767
- Goldsweig AM, Tak HJ, Chen LW, et al. The evolving management of aortic valve disease: 5-year trends in SAVR, TAVR and medical therapy. Am J Cardiol 2019; 124: 763-771. DOI: 10.1016/j.amjcard.2019.05.044
- Sherwood MW, Vora AN. Challenges in aortic stenosis: Review of antiplatelet/anticoagulant therapy management with transcatheter aortic valve replacement (TAVR): TAVR with recent PCI, TAVR in the patient with atrial fibrillation, and TAVR thrombosis management. Curr Cardiol Rep 2018; 20: 130. DOI: 10.1007/s11886-018-1073-9
- Dangas GD, Tijssen JGP, Wohrle J, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med 2020; 382: 120-9. DOI: 10.1056/NEJMoa1911425
- Park DW. Anticoagulation versus dual antiplatelet therapy for preventing leaflet thrombosis and cerebral embolization after transcatheter aortic valve thrombosis (ADAPT-TAVR). Retrieved from: https://clinicaltrials.gov/ct2/show/record/NCT03284827?view=record
- Collet JP, Berti S, Cequier A, et al. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: The randomized ATLANTIS trial. Am Heart J 2018; 200: 44-50. DOI: 10.1016/j.ahj.2018.03.008
- Van Mieghem NM, Unverdorben M, Valgimigli M, et al. Edoxaban versus standard of care and their effects on clinical outcomes in patients having undergone transcatheter aortic valve implantation in atrial fibrillation - Rationale and design of the ENVISAGE-TAVI AF trial. Am Heart J 2018; 205: 63-9. DOI: 10.1016/j.ahj.2018.07.006
- Seeger J, Gonska B, Rodewald C, Rottbauer W, Wohrle J. Apixaban in patients with atrial fibrillation after transfemoral aortic valve replacement. J Am Coll Cardiol Intv 2017; 10: 66-74. DOI: 10.1016/j.jcin.2016.10.023
- Geis NA, Kiriakou C, Chorianopoulos E, Uhlmann L, Katus HA, Bekeredjian R. NOAC monotherapy in patients with concomitant indications for oral anticoagulation undergoing transcatheter aortic valve implantation. Clin Res Cardiol 2018; 107: 799-806. DOI: 10.1007/s00392-018-1247-x
- Pan KL, Singer DE, Ovbiagele B, et al. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: A systematic review and meta-analysis. J Am Heart Assoc 2017; 6:e005835. DOI: 10.1161/JAHA.117.005835
- Briasoulis A, Inampudi C, Akintoye E, Alvarez P, Panaich S, Vaughn-Sarrazin M. Safety and efficacy of novel oral anticoagulants versus warfarin in medicare beneficiaries with atrial fibrillation and valvular heart disease. J Am Heart Assoc 2018; 7: e008773. DOI: 10.1161/JAHA.118.008773
- Hampton ML, Tellor KB, Armbruster AL, Theodos G, Schwarze MW. Evaluation of the safety and effectiveness of direct-acting oral anticoagulants in patients with atrial fibrillation and coexisting valvular heart disease. Am J Cardiovasc Drugs 2020; Epub ahead of print. DOI: 10.1007/s40256-020-00398-x
Contents
Columns
Public Education & Awareness Outreach Publication Subcommittee
Attention ASHP Pharmacist Members
Professional Affairs - CPE Opportunity!
Features
ICHP's Newest Affiliate Leaders
Opioid Task Force - CPE Opportunity
College Connection
Midwestern University Chicago College of Pharmacy
Rosalind Franklin University College of Pharmacy
Southern Illinois University Edwardsville School of Pharmacy
University of Illinois Chicago College of Pharmacy
More
Pharmacy Action Fund Contributors
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.
