Official Newsjournal of the Illinois Council of Health-System Pharmacists
Opioid Task Force - CPE Opportunity!
Common Questions about the Illinois Prescription Drug Monitoring Program
Feature Article
by Chris Herndon, PharmD, BCACP; Professor, School of Pharmacy, Southern Illinois University Edwardsville, Edwardsville, IL and Sarah Pointer, PharmD; Clinical Director of the Prescription Monitoring Program, Bureau of Pharmacy and Clinical Support Services, Springfield, IL
How has the ILPMP impacted opioid overdose rates in Illinois?
As the million-dollar question, the impact of the ILPMP on opioid mortality in Illinois may be the most difficult question to answer. Several studies have shown a direct correlation between the enactment of prescription drug monitoring program laws and opioid prescribing rates.1–3 Based on the Centers for Disease Control and Prevention (CDC) 2019 Surveillance Report of Drug-Related Risks and Outcomes, Illinois has among the lowest rates of long-acting or extended release opioid prescriptions and among the lowest rate of high-dosage opioid prescribing (defined as morphine milligram equivalents greater than 90 mg daily).4 Unfortunately, Illinois ranks 14th in the nation for age-adjusted drug overdose deaths per 100,000 population. While the ILPMP and other risk mitigation practices has undoubtedly reduced prescription substance abuse and diversion, a swift and unprecedented shift to synthetic and semi-synthetic illicit opioids has driven the steady increase in mortality rates. However, in 2018, Illinois realized its first decrease in opioid mortality rate with a lookback period of 5 years.
Access to prescription drug monitoring program data has long been a topic of great contention. The earliest state drug monitoring programs were housed within various agencies of law enforcement (e.g. California & Hawaii). The Illinois prescription monitoring program was the first to be housed within a state Department of Health. This had significant ramifications on the privacy of personal health data and the access to such data by law enforcement. In Illinois, law enforcement may only request indirect access for active cases under investigation.
Utilizing a patient’s first name, last name, and date of birth is a good place to start for most searches. However, the database is only as accurate as the prescription information uploaded to the system. For instance, if you were to search Christopher Herndon, you may miss data that was uploaded as Chris Herndon. For this reason, I usually recommend using the first four letters of the last name and the first three letters of the first name to reduce the risk of missing results due to variations in spelling of the name. The ILPMP also allows users to simultaneously search other state monitoring programs. Illinois currently has agreements to share data with 22 other states, including all bordering states (and Missouri’s St. Louis County PDMP).
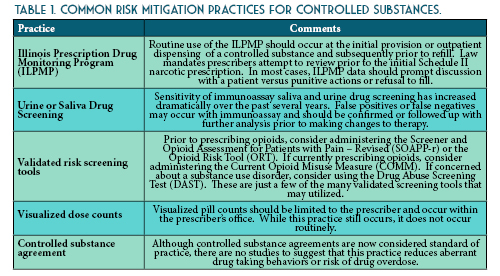
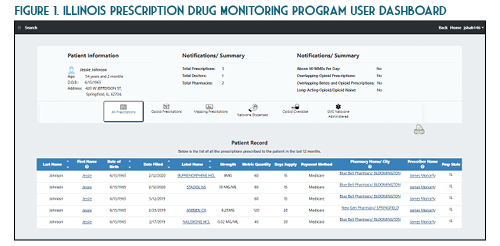
Once you verify your search results, the new ILPMP dashboard provides a valuable summary of information such as the total prescriptions, total prescribers, and total pharmacies for that patient within the last year. You can also quickly identify if that patient currently has prescriptions with a cumulative morphine equivalent daily dose (MEDD) of over 90 mg, currently has overlapping opioid prescriptions or overlapping opioid and benzodiazepine prescriptions, or if a patient has received a long-acting opioid while previously considered opioid-naïve (See Figure 1). A patient may be considered opioid-naïve if they have been on less than 60 mg of oral morphine, or its equivalent, for a duration of seven days or less. One newer feature that is perhaps the most useful is the “mapping prescriptions” function, which allows for geo-mapping of patient address, prescriber address, and pharmacy address (See Figure 2). Keep in mind that all this data represents a point on the map and doesn’t necessarily confirm substance abuse or diversion. Occasionally information in the ILPMP can be incorrect, therefore it is always recommended that prescribers and dispensers confirm the available ILPMP information with the patient.
In the State of Illinois, a prescriber (or their designee) must document an attempt to access the ILPMP prior to providing a prescription for an initial Schedule II narcotic prescription (720 ILCS 570/314.5). At this time pharmacists are not required to document an attempt to access the ILPMP. However, based on the pharmacist’s “corresponding liability” under the Federal Controlled Substances Act, this practice is highly encouraged. While the law requires accessing the ILPMP only for the initial prescription, in practice, this should be performed (and documented) prior to each prescription from a patient safety and medico-legal standpoint. The requirement to document an attempt to access the ILPMP prior to issuing an initial Schedule II opioid prescription does not apply to the inpatient setting, patients receiving active oncology treatment, palliative care / hospice patients, or patients receiving a seven day or less supply from an emergency department.
On January 1st, 2018, Illinois Public Act 100-0564 mandated that all electronic health record (EHR) systems utilized within Illinois interface directly with the ILPMP on or before January 1st, 2021. The integration of the PMP into the electronic health record will enable the prescriber to view the PMP data without leaving their workflow and logging into the ILPMP website. Utilizing this integration, also known as PMPnow, is anticipated to save time and money. The PMPnow integration can be requested through the ILPMP at no cost to the health-system or pharmacy, although the EHR or pharmacy software vendor may charge the system for upfront costs associated with establishing the ILPMP connection. Currently ILPMP is integrated with approximately 250 pharmacies. Prescribers and pharmacists should work with their EHR and pharmacy software vendor to ensure they are meeting stale law requirements. The connection between the ILPMP and your respective EHR is encrypted to ensure patient confidentiality.
Yes. If you are a prescriber or pharmacist directly involved in the care of a patient, then accessing the ILPMP data and communicating that data with another professional directly involved in the care of the patient would fall under the HIPAA definition of “treatment.” For instance, if you are a pharmacist and note that one of your patients is receiving alprazolam from his psychiatrist and his primary care physician, calling both prescribers would be considered reasonable under the HIPAA definition. There is some ambiguity, however, if you do not have an established relationship with the patient. Perhaps a new patient comes to your pharmacy with a prescription for hydrocodone. You refuse to fill the prescription due to concerning information on the ILPMP. Calling the prescribers of this patient may not be covered under the HIPAA definition of “treatment.”
You are a community pharmacist in a large retail chain. A patient well known to you approaches the counter with a prescription for fentanyl transdermal patch 75 mcg/hour. You review his prescription profile and note a routine monthly prescription for oxycodone / acetaminophen oral tablets 5-325 mg with instructions to take 1 tablet PO every 8 hours as needed for severe pain. While you know a prior authorization will be required for the patient’s prescription insurance, you ask your pharmacy student what they would like to do.
The student recommends first asking the patient if they have been on the fentanyl patch prior and if so, what strength and how long ago. The patient denies prior experience with fentanyl patches. You ask your stellar student if this patient is opioid-naïve or opioid tolerant. The student notes that the patient routinely fills the oxycodone tablets each month, assuming that they use the three allowed doses each day. This would be 15 mg of oral oxycodone which is equivalent to approximately 20 mg of oral morphine equivalents daily (MEDD). While the patient has been on this therapy for several years, the MEDD is less than 60 mg, which means this patient should be classified as opioid-naïve. The safety of the fentanyl patch for this patient should be questioned. You and your student log on to the ILPMP and note that this patient has been receiving oral controlled release morphine 60 mg dosed every 12 hours from the same prescriber, but it is filled at a different pharmacy. This would place the patient in the opioid-tolerant category, but the different pharmacy certainly raises a red flag. You mention this when you call the prescriber. Before the patient leaves, the student asks, “what about naloxone?” and suggests to the pharmacist that if the patient does not have a dose of naloxone available at home, they should consider obtaining one per the state-wide standing order while providing the standardized procedures for administration.
You are a hospital pharmacist working in the emergency department. Your hospital has recently integrated PMPnow into your electronic health record. A patient presents for uncontrolled low back pain due to a fall and is requesting something for severe pain. You review this patient’s ILPMP record and note that they are routinely using fentanyl transdermal patches. A urine drug screen is positive for an “opiate.” The emergency physician comes by on her way to see the patient and stops to ask you your thoughts.
First and foremost, the ILPMP should be used to improve patient care, not as punitive action. You are to be commended for reviewing the ILPMP. However, because this patient falls under one of the exempt categories, documenting the attempt to access the ILPMP is not legally-mandated should you choose to send this patient out with a prescription for an opioid analgesic. The other concern is the positive drug screen for “opiate.” Traditionally immunoassay urine drug screens are not sensitive for synthetic or semisynthetic opioids unless specifically stated. The first conclusion here would be the patient is using an illicit opioid. That certainly is a distinct possibility, but an additional consideration could be that this patient received an opioid analgesic in another emergency department or hospital recently. This would not be reported to the ILPMP.
The Illinois Prescription Drug Monitoring Program is a valuable tool for prescribers, pharmacists, and pharmacy technicians. Routine review of the ILPMP is essential for improved clinical decision making, safer opioid prescribing, and improved patient outcomes while reducing opioid misuse, abuse and overdose. Both prescribers and pharmacists may designate up to three licensed designees to query the database on their behalf. Pharmacists should be prepared to discuss ILPMP findings or concerns with both patients and prescribers. Pharmacy technicians should be prepared to discuss ILPMP findings with a pharmacist.
Sarah Pointer, PharmD
401 North Fourth Street
Springfield, IL 62702
https://www.ilpmp.org/index.php
https://www.ilpmp.org/PMPnowRegistration.php
- Gugelmann HM, Perrone J. Can prescription drug monitoring programs help limit opioid abuse? J Am Med Assoc. 2011;306(20):2258-2259. doi:10.1001/jama.2011.1712.
- Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13(3):434-442. doi:10.1111/j.1526-4637.2012.01327.x.
- Fink DS, Schleimer JP, Sarvet A, et al. Association between prescription drug monitoring programs and Nonfatal and Fatal Drug Overdoses: A Systematic Review. Ann Intern Med. 2018;168(11):783-790. doi:10.7326/M17-3074.
- Centers for Disease Control and Prevention. 2019 Annual surveillance report of drug-related risks and outcomes — United States surveillance special report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published November 1, 2019. Accessed 2020 Apr 2020 from https://www. cdc.gov/drugoverdose/pdf/ pubs/2019-cdc-drug-surveillance? report.pdf.

Contents
Columns
Features
2020 Spring Meeting Poster Presentations
Opioid Task Force - CPE Opportunity!
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University College of Pharmacy
Rosalind Franklin University of Medicine and Science College of Pharmacy
Southern Illinois University Edwardsville (SIUE) - School of Pharmacy
University of Illinois at Chicago College of Pharmacy
More
ICHP Pharmacy Action Fund (PAC)
Board of Directors, Student Society Presidents & Affiliates
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.
