Print This Article
Educational Affairs
ASA for Primary Prevention - A Worthy Topic for Shared-Decision Making
by By Regina Arellano, Pharm.D., BCPS Assistant Professor Midwestern University Chicago College of Pharmacy and Jaini Patel, Pharm.D., BCACP Assistant Professor Midwestern University Chicago College of Pharmacy
In 1985 aspirin, also known as acetylsalicylic acid (ASA), was approved by the Food and Drug Administration (FDA) for secondary prevention of cardiovascular disease (CVD). Its benefit in reducing CVD morbidity and mortality in patients with occlusive CVD events, including subsequent coronary heart disease (CHD), has consistently proven to outweigh risks of major bleeding associated with its long-term use. However, its role in primary prevention is less clear and necessitates meticulous evaluation and shared decision making to determine realistic benefit-to-harm ratio. Due to lack of clear benefit-to-harm ratio from ASA use for primary prevention, consensus regarding its use also varies. Nonetheless, it has been widely studied and utilized as one of the cornerstone pharmacological interventions for primary prevention of CVD.1
Long-term ASA therapy, at lower doses in the range of 75 to 100 mg daily, has been associated with a reduction in CV events. The subgroup analyses from the 2002 Antithrombotic Trialists Collaboration (ATTC) meta-analysis indicated that ASA appears to be equally effective for the prevention of CVD at doses between 75 and 325 mg daily.2 Hence, for primary prevention, ASA doses of ≤100 mg/day should be used to minimize risk of bleeding. This is in congruence with routine use of ASA 81 mg/day dose in clinical practice.
The most concerning adverse effect of ASA is major bleeding, defined as bleeding which requires hospitalization with or without transfusion. The most common type of ASA-associated bleeding is gastrointestinal (GI) bleeding, and it is rarely fatal.3 Inhibition of cyclooxygenase-1 (COX-1) enzyme increases risk of upper GI bleeding, however, this risk is dose-dependent with ASA therapy and can be minimized with use of lower doses. The incidence of major bleeding is likely to be somewhat higher in the general population than among participants in randomized trials. The U.S. Preventive Services Task Force (USPSTF) report on the use of ASA for the primary prevention of CVD and cancer suggested that increasing age, male sex, and diabetes increased the risk for major bleeding with ASA therapy.4 According to 2008 American College of Cardiology/American College of Gastroenterology/American Heart Association (ACC/ACG/AHA) guidelines, risk factors for GI toxicity from non-steroidal anti-inflammatory drugs (NSAIDs) such as ASA include history of ulcer disease or ulcer complication, concurrent use of antiplatelet, anticoagulant, or glucocorticoid therapy, age ≥60 years, dyspepsia or gastroesophageal reflux disease (GERD) symptoms. In patients who have an episode of major bleeding while taking ASA for primary prevention, it should be determined if the risk of recurrent bleeding outweighs the benefits of long-term use.
Identification of individual risk factors, application of atherosclerotic cardiovascular disease (ASCVD) assessment tools, and initiation of evidence-based pharmacotherapy permits clinicians to approach primary prevention effectively in high ASCVD risk individuals. An ASCVD risk score of >20% confers high CVD risk while a <5% confers low CVD risk. Clinicians should use clinical judgement and other tools to sufficiently assess ASCVD risk in this lower risk population (e.g., family history of premature ASCVD, high-sensitivity C-reactive protein [hs-CRP], coronary artery calcium measurement). After a comprehensive CVD risk assessment, it is important to conduct an in-depth discussion and shared decision making about lifestyle modifications and management of other CVD risk factors including dyslipidemia, diabetes, and hypertension. As an example, for a given patient with ASCVD risk >20%, not on statin therapy, and with > 10 years duration of type 2 diabetes and A1c >10%, the first step would be to optimize control of underlying risk factors.
In order to implement evidence-based guideline recommendations into clinical practice, clinicians should evaluate practice-changing primary literature. Starting in 1988, clinical trials sought to evaluate the potential benefit of aspirin for primary prevention of CVD.5 Several meta-analyses have been conducted to collectively evaluate outcomes of major landmark trials published over the past 3 decades (1988 to 2018) to identify the benefits of ASA in primary prevention. The collated evidence from these trials with follow-up range of 3.8-10 years indicated that ASA therapy results in (1) none to very small reduction in all-cause or CVD mortality [high-quality evidence], (2) reduction in non-fatal MI over 10 years [moderate-quality evidence], and (3) none to very small reduction in non-fatal stroke over 10 years.
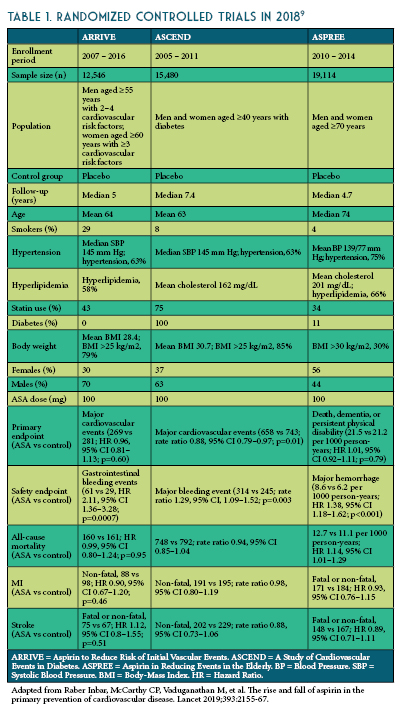
The three most recent trials published in 2018 (The Aspirin to Reduce Risk of Initial Vascular Events [ARRIVE], A Study of Cardiovascular Events in Diabetes [ASCEND], and The Aspirin in Reducing Events in the Elderly [ASPREE]) are more reflective of modern preventative practices including blood pressure control, smoking cessation, and cholesterol reduction (See Table 1).6-8
The ARRIVE enrolled 12,546 patients ≥ 55 years (men) or ≥60 years (women) at a moderate risk of CHD (≥ 3 cardiovascular risk factors: dyslipidemia, current smoker, high blood pressure, positive family history of CVD).6 In ARRIVE, ASA use in the intermediate-risk population (~15% in 10 years) did not reduce the composite outcome of first MI, stroke, CV death, unstable angina or transient ischemic attack, while GI bleeding events were more than twice as likely with ASA use.
The ASCEND trial enrolled 15,480 patients with diabetes, age >40 years, and no known CVD.7 In ASCEND, ASA use in patients with diabetes led to 12% relative reduction in non-fatal MI, stroke, transient ischemic attack or vascular death (number needed to treat=91) excluding intracerebral hemorrhage, while major bleeds were 29% more likely (number needed to harm=112). Approximately half the excess of bleeding was in the GI tract, with approximately one-third in the upper GI tract.
The ASPREE trial enrolled 19,114 healthy older adults (>70 years old, >65-year-old in Hispanic or African American patients) without a history of CVD, cerebrovascular disease, dementia, or any other chronic condition that would likely limit survival to less than 5 years.8 In ASPREE, ASA use in healthy elderly patients showed no reduction in MI or ischemic strokes, however, there was a substantial, progressive increase in major hemorrhage. In addition, there was an increase in all-cause death in the ASA group, mainly due to increased cancer deaths, specifically.
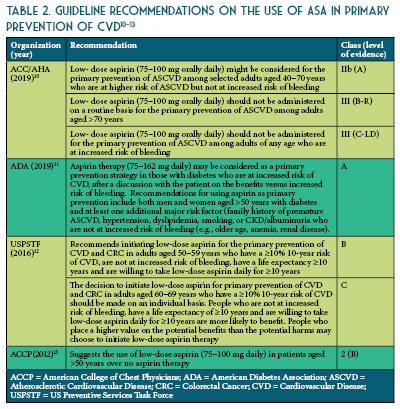
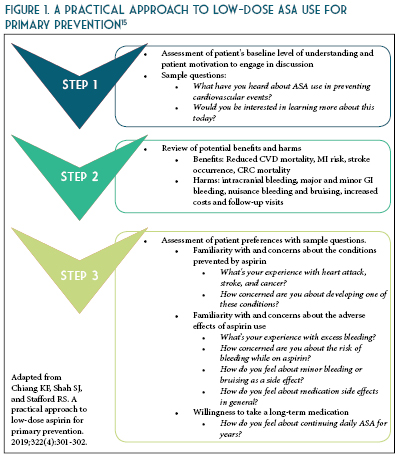
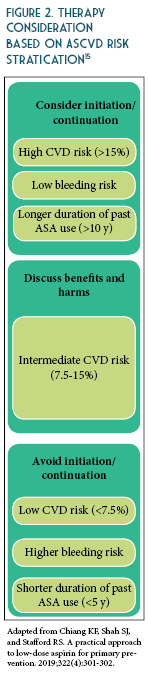
The updated recommendations from ACC/AHA based on recent evidence is as follows: consider ASA use for primary prevention in adults 40-70 years of age at higher risk of CVD (ASCVD score >10%) but not at an increased risk of bleeding.10 Routine use of ASA is not recommended in adults >70 years of age or any age with increased risk of bleeding (See Table 2).10-13 To date, there is lack of evidence to justify use of ASA of primary prevention in patients with the highest CVD risk (>20%), those of African American descent, and those with uncontrolled co-morbidities that increase CVD risk. Therefore, more research is needed to identify patients with high CVD risk and acceptable bleeding risk for whom taking a once daily affordable therapy such as a low-dose ASA is worth considering. The benefits were more pronounced when estimated 10-year ASCVD risk was >7.5%.14 These findings suggest that the decision to use aspirin for primary prevention should be tailored to the individual patient based on estimated ASCVD risk (See Figures 1 and 2) and perceived bleeding risk, as well as patient preferences regarding types of events prevented versus potential bleeding caused. When aspirin is used for primary prevention, a low dose (<100 mg/day) should be recommended.
References
- Miner J, Hoffhines A. The discovery of aspirin’s antithrombotic effects. Tex Heart Inst J 2007;34:179–186.
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71.
- Selak V, Kerr A, Poppe K, et al. Annual Risk of Major Bleeding Among Persons Without Cardiovascular Disease Not Receiving Antiplatelet Therapy. JAMA 2018; 319:2507.
- Whitlock EP, Burda BU, Williams SB, et al. Bleeding Risks With Aspirin Use for Primary Prevention in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164:826.
- Miedema MD, Huguelet J, Virani SS. Aspirin for the primary prevention of cardiovascular disease: in need of clarity. Curr Atheroscler Rep 2016;18:4.
- Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018; 392:1036.
- ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529.
- ASPREE
- Raber Inbar, McCarthy CP, Vaduganathan M, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet 2019;393:2155-67.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. JACC 2019;74:e177-232.
- ADA. Cardiovascular disease and risk management. Diabetes Care 2019;42(1):103.
- Bibbins-Domingo, K, on behalf of the U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventative Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836-845.
- Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e637S-e668S.
- Abdelaziz HK, Saad M, Pothineni N, et al. Aspirin for primary prevention of cardiovascular events. JACC 2019;73:2915-2929.
- Chiang KF, Shah SJ, and Stafford RS. A practical approach to low-dose aspirin for primary prevention. JAMA 2019;322(4):301-302.