Print This Article
Educational Affairs
Implementing Anticoagulation Dosing Guidance and Drug-Drug Interaction Procedures
by By Cody C. Anderson, PharmD Long Term Care Consultant Pharmacist, Omnicare; Megan Grischeau, PharmD, BCACP VISN12 Clinical Pharmacist, Anticoagulation Hub – Midwest Region 2016-2019 Captain James A. Lovell Health Care Center PGY2 Ambulatory Care Residency Program Director Veterans Health Administration Integrated Service Network (VISN12)
Introduction
Anticoagulation management is a process that has largely been handled by pharmacists. Data has shown that a pharmacist-driven anticoagulation management is no less effective than physician-managed with improved patient satisfaction.1-2 Significant consideration is needed when evaluating an anticoagulation regimen for safety and efficacy, including such factors as appropriate dosing, monitoring, and drug interactions. At the Captain James A. Lovell Federal Health Care Center (FHCC), processes for notification of warfarin drug-drug interactions and low molecular weight heparin (LMWH) dose rounding were standardized and implemented to maintain consistency across providers and to provide the safest care possible to the Veterans.
FHCC is a very unique organization, being the first and only combined Veterans Affairs (VA) and Department of Defense (DoD) facility. As a general statement, VA facilities serve only Veterans, while DoD facilities serve mainly active duty personnel, their dependents, and retirees. The process of merging two facilities into one has not been seamless. One barrier in particular is that both the VA and DoD utilize different computer systems; each with one system for electronic order entry by the provider and another for processing by the pharmacy. This means that medication orders entered through the VA systems will not cross over into the DoD computer system, and vice versa. In addition, some VA patients may have certain eligibilities, which require that they receive their medications through the DoD system, even if they are not receiving care from DoD providers. Unlike some private facilities where drug interaction alerts might seem customary, this joint VA/DoD venture has the aforementioned complicating factors to overcome. The following will describe the processes for enhancing patient safety through newly standardized processes.
Warfarin Drug-Drug Interaction Protocol
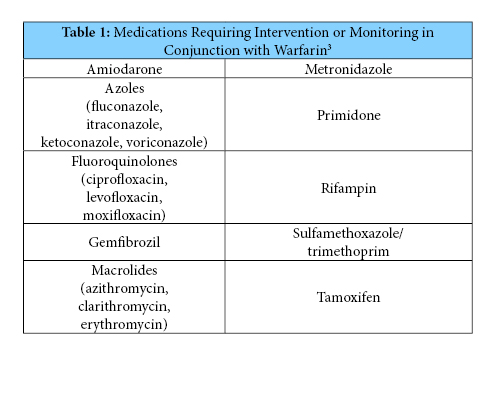
Warfarin has many drug-drug interactions, with some more likely to cause significant clinical interactions than others. Initiation or dosage adjustments of the medications in Table 1 were selected as being significant enough to warrant action.
Until recently, FHCC did not have a formal policy in place guiding inpatient and outpatient pharmacy on how to handle warfarin drug-drug interactions. At the FHCC, all warfarin is managed by clinical pharmacy specialists (CPS). Over the past two years, outpatient anticoagulation management responsibilities have shifted from the specific team’s CPS to a telephone based Centralized Anticoagulation Clinic (AC) model. Because the clinic may be staffed by different CPS on various days of the week, it was challenging for the outpatient pharmacy staff to identify which CPS to contact with specific concerns. For this reason, a Standard Operating Procedure (SOP) regarding the management of warfarin drug-drug interactions was necessary to improve patient safety.
This SOP applies to outpatient prescriptions dispensed by outpatient pharmacy, or outpatient prescriptions that are dispensed by inpatient pharmacy when the outpatient pharmacy is closed. The processing pharmacist or technician is responsible for identifying patients prescribed warfarin who received a new prescription or dosage adjustment of one of the aforementioned medications. If a patient receives VA care, the processing pharmacist will notify the AC CPS via a newly created email group with an encrypted message; or for DoD patients, the processing pharmacist will email the CPS directly as these patients were not included in the centralized model.
Clinical pharmacy specialists are responsible for monitoring the email account, evaluating critical drug interactions within one business day, and intervening as deemed appropriate. A few factors that the clinical pharmacist must take into consideration when evaluating a drug-drug interaction may include: length of therapy of the interacting drug, current or most recent INR, a patient’s risk for bleeding or thrombosis, and ability for patient follow-up monitoring. Interventions may include warfarin dosage adjustment, changing the follow-up appointment date, ordering labs, contacting the prescribing provider, or other interventions based on the CPS’s judgment. CPS ensure that a follow-up clinic, telephone, or lab appointment is scheduled and dates are relayed to the patient. Appropriate documentation of medication review and any needed interventions are completed in the patient’s electronic medical record.
Education was provided to the processing pharmacists and CPS upon implementation of the SOP and any questions they may have had were answered. The only question after training was knowing which pharmacist provider to email, and this was further clarified. In February 2018, this procedure was approved and posted to the pharmacy shared drive to allow for accessibility by all pharmacists. Continued follow-up from the anticoagulation clinic manager will be needed to ensure pharmacists implement and follow the procedures appropriately, including monitoring the VA email account and asking for feedback from the DoD CPS regarding ease of process.
With patient safety being a top priority, this was an area that necessitated change. Previously, processing pharmacists were not required to notify any CPS of potential warfarin drug-drug interactions. This process will greatly improve patient safety, as in the past the process relied on the patient to contact the clinic with medication changes.
LMWH Dosing Guidance
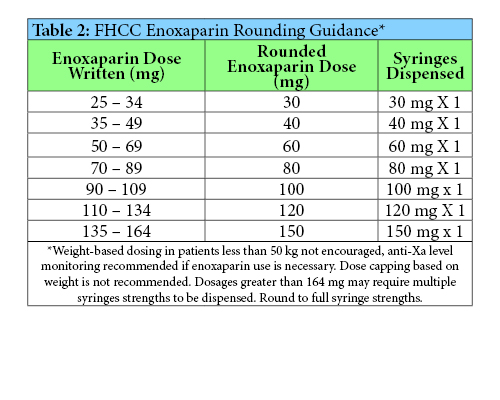
Outpatient dosing of LMWH can be challenging. A patient’s actual body weight is used to calculate the dose, either as a 1 mg/kg or 1.5 mg/kg dose for enoxaparin, and 150 units/kg or 200 units/kg for dalteparin.4-5 However, given that LMWH syringes are only available in certain dosages with select syringes sizes having marked graduation, a patient’s dose will frequently need to be rounded up or down to reach the nearest syringe size. Some providers or institutions will instruct patients to “waste” part of a syringe to reach a specific dose, but this can lead to patients administering incorrect or inconsistent dosages. Relying on patients to waste the correct amount was identified as less ideal and offered an opportunity to standardize care and reduce the potential for medication errors. As seen in Tables 2 and 3, a provider can quickly and easily find the recommended dosage and available syringe strengths based on a patient’s calculated weight-based dose.
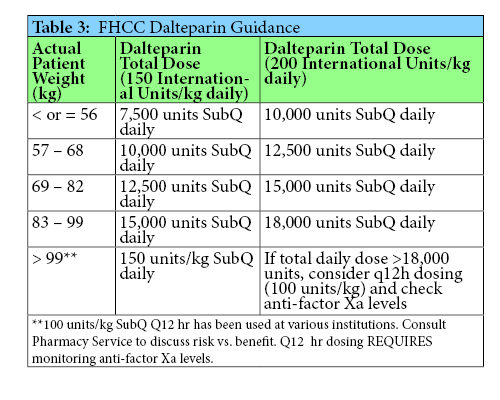
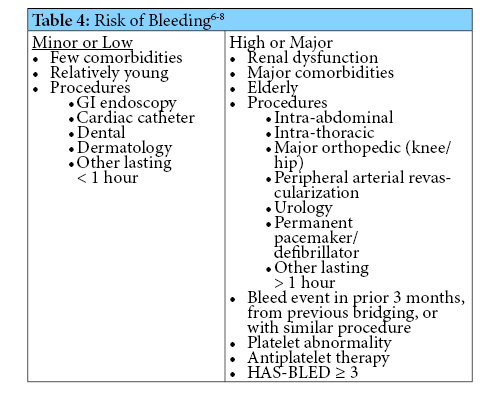
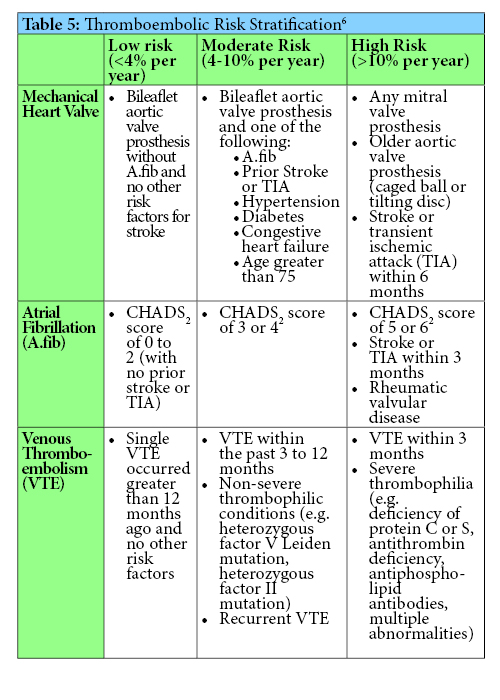
A provider may choose to round up or down based on the patient’s individual risk of bleeding or thrombosis. Table 4 provides situations in which a patient may be classified as a low or high bleed risk, and Table 5 classifies thrombotic risk based on specific indications and risk factors. The LMWH recommendations were not implemented as a protocol that must be followed, but created as a reference to help guide providers.
It is important to note that patients should have baseline labs performed when using either LMWH formulation, including a complete blood count and serum creatinine. Anti-Xa activity monitoring may also be recommended in specific situations such as extremes in body weight, renal impairment, pregnancy, or for patients receiving chronic use for venous thromboembolism (VTE) prophylaxis in the presence of a mechanical heart valve.4-5,9
Conclusion
Since these processes were recently adopted, it is too soon to know their full impact. It can be difficult to change previous practice patterns, thus feedback from pharmacists and providers will be welcomed to maximize the efficacy and efficiency of the guidance and SOP. Time-in-therapeutic-range reports can be accessed in the VA data warehouse to potentially assess safety and efficacy of the process. However it is possible that some patients may not have an INR for four weeks following the drug interaction. At that time, the interacting drug may already be eliminated from the patient’s system and INR could have already returned to normal. Therefore, evaluation of the process may require generating reports of the above drug-drug interactions to assess if there was corresponding documentation in the patient’s medical record. Reassessment of the warfarin drug-drug interaction SOP may require re-education of pharmacy staff depending on activity of the CPS email group regarding interactions.
References
Young S, Bishop L, Twells L, et al. Comparison of pharmacist managed anticoagulation with usual medical care in a family medicine clinic. BMC Family Practice 2011;12:88.
Zhou S, Sheng X, Xiang Q, et al. Comparing the effectiveness of pharmacist-managed warfarin anticoagulation with other models: a systematic review and meta-analysis. J Clin Pharm Ther 2016;41:602-11.
Bungard T, Yakiwchuk E, Foisy M, et al. Drug interactions involving warfarin: practice tool and practical management tips. Can Pharmaceut J 2011;144(1):21-25.
Lovenox® [package insert]. Bridgewater, NJ: Sanofi-Aventis; October 2017.
Fragmin® [package insert]. New York, NY: Pfizer Inc; June 2017.
Douketis J, Spyropoulos A, Spencer F, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians evidence-based clinical practice guidelines (9th Edition). Chest 2012;141:326S-350S.
Lip GY, Frison L, Halperin J, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2011 Jan 11;57(2):173-80.
Doherty J, Gluckman T, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with non valvular atrial fibrillation. J Am Coll Cardiol 2017 Feb 21;69(7):871-898.
Duhl A, Paidas M, Ural S, et al. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromboembolism and adverse pregnancy outcomes. AJOG Nov 2007;457:e1-57.e21.