Official Newsjournal of the Illinois Council of Health-System Pharmacists
Educational Affairs
The Effectiveness and Safety of Various Abuse Deterrent Formulations of Oxycodone: A Systematic Review
by Alexander Heinz, PharmD Candidate, Roosevelt University College of Pharmacy; Julia Gilbert, PharmD Candidate, Roosevelt University College of Pharmacy; Gerald Cavanagh, PharmD Candidate, Roosevelt University College of Pharmacy; Abby A. Kahaleh, BPharm, MS, PhD, MPH, Associate Professor, Roosevelt University College of Pharmacy
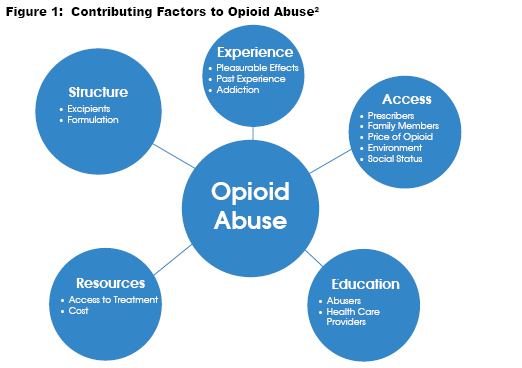
Research over the past decade has extensively examined drug structures to address the emerging opioid pandemic. Opioid analgesics with abuse-deterrent properties can help prevent abuse via various routes of administration that require cutting, crushing, or other ways of manipulating the formulations. There are three methods utilized to create abuse-deterrent formulations (ADFs) are described below:
- Technology-Based ADF’s: One category of abuse deterrent formulations is technology-based. These formulations use proprietary manufacturing that utilizes unique polymers, inactive beads, and excipients to maintain the original pharmacokinetic properties or “gel” upon crushing or dissolving, which prevents abuse in all routes of administration except the oral route.1
- Opioid Antagonist ADF’s Another category utilized is the addition of opioid antagonists, either naloxone or naltrexone to the formulation. Opioid antagonists compete and displace opioids at opioid receptor sites and can be formulated in dosage forms to release when a medication in inappropriately utilized, such as crushing, or with a route of administration that favors the opioid over the antagonist.2
- Aversive Excipients ADF’s The third category of abuse deterrent formulations consists of opioids with a particular aversive excipient, such as niacin.3 This excipient gives the medication an unpleasant, unwanted side effect when used in excess and therefore, helps prevent abuse with the formulation.
The primary objective of this study was to review the evidence for the safety and effectiveness of abuse deterrent formulations (ADF) of oxycodone. The secondary objective was to evaluate the potential of the ADF to prevent opioid abuse in American adults.
Methods
A systematic review method was utilized to conduct this research. The studies included in the systematic review were randomized controlled trials in adult participants who have a history of prescription and/or nonprescription opioid use. The interventions included the administration of various abuse deterrent oxycodone formulations compared to placebo and current non-abuse deterrent formulations.
The inclusion criteria for the systematic review was inclusion of the terms “oxycodone,” “abuse deterrent,” “opioid,” “abuse resistant,” and “randomized.” The studies were all published in English and conducted in North America. Exclusion criteria were studies not published in English, conducted outside of North America, not relevant to the safety and efficacy of abuse deterrent formulations, and not evaluating oxycodone.
Data Collection
Three review authors independently assessed all the titles and abstracts identified as a result of the search strategy. Twenty-seven articles were collected on the search, 12 fit inclusion/exclusion criteria. Thirteen articles were not relevant to the study subject, as they were found to later fit the exclusion criteria and 2 publications were found to be published presentations. Three types of ADFs were evaluated in the data collected: opioid antagonist formulations, technology-based formulations, and aversive excipient-based formulations.
The three abuse deterrent formulation categories were ranked on an efficacy scale of 1-to-3, with 1 meaning highly effective and 3 meaning least effective. The efficacy was evaluated based on patient reported feeling, adverse events, and potential adherence. Of the studies included, nine were categorized as technology-based formulations, two as opioid antagonist formulations, and one as aversive excipient-based formulations. The previous drug effects were compared with adverse events to indicate how effective treatments were. In the rare occasions that the researchers disagreed on the ranking of the articles, they discussed the discrepancies until a consensus was reached.
Results
The following three tables describe the articles that were included in the systematic review. All three categories had similar efficacy results and were shown to prevent abuse. However, their safety profiles and types of abuse they prevented differed slightly.
By reducing the abuse potential from non-medical routes of administration, especially those seen with heroin use, rates of abuse may be reduced. Although educating patients remains the most important step in reducing the epidemic of opioid abuse and overdose, studying additional ways to deter and reduce abuse can be extremely helpful in furthering reducing the abuse potential.4 Figure 2 illustrates the efficacy ranking of the three categories of ADFs.
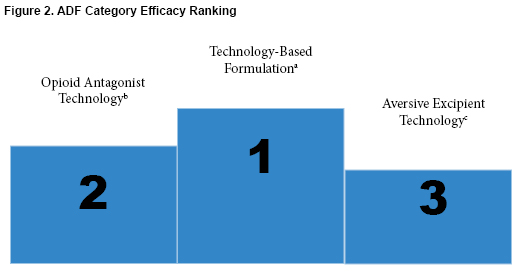
- Technology-based formulations had the highest efficacy, safety, and overall ability to be used by patients. It can prevent opioid abuse by oral route and non-oral routes of administration and has a safety profile similar to currently approved opioid analgesics.
- Opioid antagonists are effective in preventing abuse, however, these are more successful in preventing abuse via non-oral routes such as intravenous or insufflation.
- Although oxycodone and niacin produced significant reduction in drug liking, the adverse event profile is a barrier to adherence.
- Wide-ranging online data for epidemiologic research (WONDER). Centers for Disease Control and Prevention. http://wonder.cdc.gov. Accessed July 2, 2016.
- Facts & Comparisons. St. Louis, MO: Wolters Kluwer Health, Inc; 2016. https://fco.factsandcomparisons.com/lco/action/doc/retrieve/docid/fc_dfc/5545901. Accessed July 2, 2016.
- Kosten TR, Haile CN. Opioid-related disorders. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, eds. Harrison's principles of internal medicine, 19e. New York, NY: McGraw-Hill Education; 2015. accesspharmacy.mhmedical.com/content.aspx?aid=1120820647.
- Siegal HA, Carlson RG, Kenne DR, Swora MG. Probable relationship between opioid abuse and heroin use. Am Fam Physician. 2003;67(5):942.
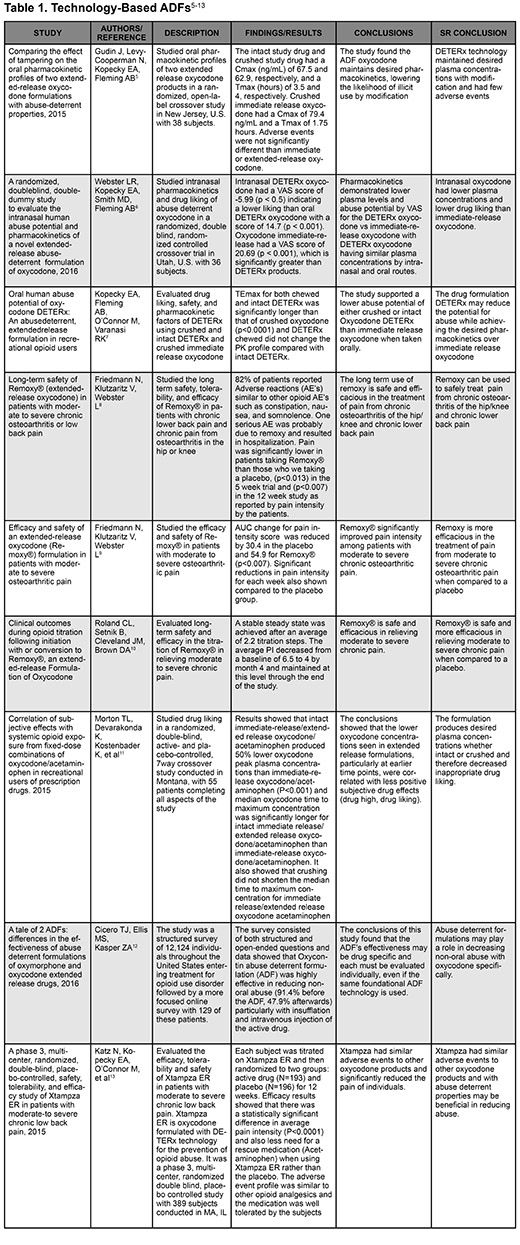
- Gudin J, Levy-Cooperman N, Kopecky EA, Fleming AB. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties. Pain Med. 2015;16(11):2142-2151.
- Webster LR, Kopecky EA, Smith MD, Fleming AB. A randomized, double-blind, double-dummy study to evaluate the intranasal human abuse potential and pharmacokinetics of a novel extended-release abuse-deterrent formulation of oxycodone. Pain Med. 2016;17(6):1112-1130.
- Kopecky EA, Fleming AB, O’Connor M, Varanasi RK. Oral human abuse potential of oxycodone DETERx: An abuse-deterrent, extended-release formulation in recreational opioid users. J Clin Pharmacol. 2017;57(4):500-12.
- Friedmann N, Klutzaritz V, Webster L. Long-term safety of Remoxy®. Pain Med. 2011;12(5):755-760.
- Friedmann N, Klutzaritz V, Webster L. Efficacy and safety of an extended-release oxycodone (remoxy) formulation in patients with moderate to severe osteoarthritic pain. J Opioid Manag. 2011;7(3):193-202.
- Roland CL, Setnik B, Cleveland JM, Brown DA. Clinical outcomes during opioid titration following initiation with or conversion to Remoxy®, an extended-release formulation of oxycodone. Postgrad Med. 2011;123(4):148-159.
- Morton TL, Devarakonda K, Kostenbader K, Montgomery J, Barrett T, Webster L. Correlation of subjective effects with systemic opioid exposure from fixed-dose combinations of oxycodone/acetaminophen in recreational users of prescription drugs. Pain Med. 2015;17(3):539-550.
- Cicero TJ, Ellis MS, Kasper ZA. A tale of 2 ADFs: Differences in the effectiveness of abuse-deterrent formulations of oxymorphone and oxycodone extended-release drugs. Pain. 2016;157(6):1232-1238.
- Katz N, Kopecky EA, O'Connor M, Brown RH, Fleming AB. A phase 3, multicenter, randomized, double-blind, placebo-controlled, safety, tolerability, and efficacy study of Xtampza ER in patients with moderate-to-severe chronic low back pain. Pain. 2015;156(12):2458-2467.
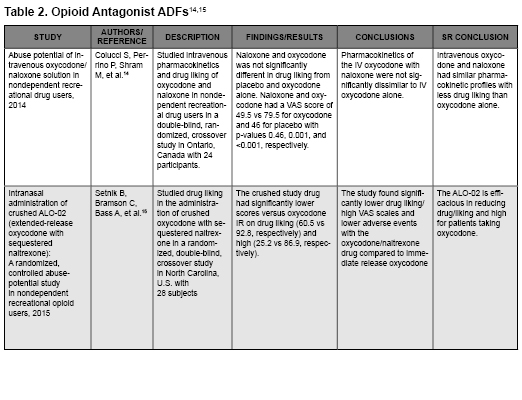
- Colucci SV, Perrino PJ, Shram M, Bartlett C, Wang Y, Harris SC. Abuse potential of intravenous oxycodone/naloxone solution in nondependent recreational drug users. Clin Drug Investig. 2014;34(6):421-429.
- Setnik B, Bramson C, Bass A, et al. Intranasal administration of crushed ALO-02 (extended-release oxycodone with sequestered naltrexone): A randomized, controlled abuse-potential study in nondependent recreational opioid users. J Clin Pharmacol. 2015;55(12):1351-1361.
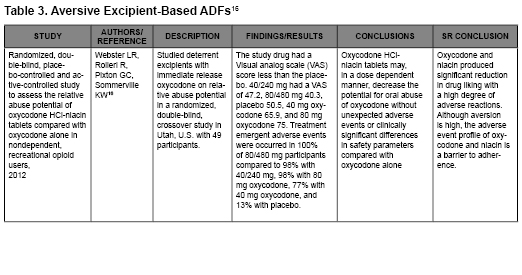
- Webster LR, Rolleri RL, Pixton GC, Sommerville KW. Randomized, double-blind, placebo-controlled and active-controlled study to assess the relative abuse potential of oxycodone HCl-niacin tablets compared with oxycodone alone in nondependent, recreational opioid users. Subst Abuse and Rehabil. 2012;3:101-113.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645.
- Topoleski CJ. RE: FDA-2014-N-1359; Development and regulation of abuse-deterrent formulations of opioid medications; public meeting. American Society of Health-System Pharmacists. 2015. https://www.ashp.org/-/media/assets/advocacy-issues/docs/ashp-comments-on-fda-development-and-regulation-of-abuse-deterrent-formulations-of-opioids.ashx?la=en. Accessed July 2. 2016.
- FDA issues draft guidelines for generic abuse-deterrent opioids. American Pharmacists Association Web site. https://www.pharmacist.com/fda-issues-draft-guidelines-generic-abuse-deterrent-opioids. Updated 2016. Accessed July 2, 2016.
- Prevention and intervention strategies to decrease misuse of prescription pain medication. American Public Health Association Web site. https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2015/12/08/15/11/prevention-and-intervention-strategies-to-decrease-misuse-of-prescription-pain-medication. Accessed July 2, 2016.
Contents
Features
Attention ASHP Pharmacist Members
Call for Posters - Deadline January 10, 2018
Columns
College Connection
Midwestern University Chicago College of Pharmacy
Roosevelt University School of Pharmacy
Rosalind Franklin University of Medicine and Science
More
Officers and Board of Directors
ICHP Pharmacy Action Fund (PAC) Contributors
Regularly Scheduled Network Meetings
Chicago Area Pharmacy Directors Network Dinner
3rd Thursday of Odd Months
5:30pm
Regularly Scheduled Division and Committee Calls
Executive Committee
Second Tuesday of each month at 7:00 p.m.
Educational Affairs
Third Tuesday of each month at 11:00 a.m.
Government Affairs
Third Monday of each month at 5:00 p.m.
Marketing Affairs
Third Tuesday of each month at 8:00 a.m.
Organizational Affairs
Fourth Thursday of each month at 12:00 p.m.
Professional Affairs
Fourth Thursday of each month at 2:00 p.m.
New Practitioner Network
Second Thursday of each month at 5:30 p.m.
Technology Committee
Second Friday of each month at 8:00 a.m.
Chicago Area Pharmacy Directors Network Dinner
Bi-monthly in odd numbered months with dates to be determined. Invitation only.